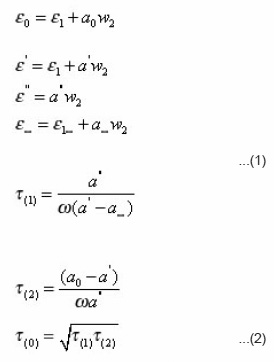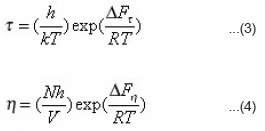Dielectric Relaxation Studies of Ethyl Formate with Primary Alcohols using Time Domain Reflectometry
S. Elangovan1* and S. Mullainathan2
*1Department of Physics, Easwari Engineering College, Ramapuram, Chennai - 600 089 (India).
2Department of Physics, A.V.C College of Engineering, Mayiladuthurai - 609 305 (India).
DOI : http://dx.doi.org/10.13005/msri/090110
Article Publishing History
Article Received on : 08 May 2012
Article Accepted on : 03 Jun 2012
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Dielectric relaxation studies of ethylformate with 1-propanol,1-butanol and 1-pentanol binary mixtures have been carried out at micro frequency range 9.36 GHZ at temperature of 303K.Different dielectric parameters like dielectric constant(ε’ ),dielectric loss (ε’’) ,Static dielectric constant (ε0) and dielectric constant at optical frequency (ε∞) have been determined. The Relaxation time (ε) has been obtained by Higasi and Cole-Cole method. The dielectric constant (τ0) and relaxation time (τ) decreased with increasing the concentration of ethylformate in alcohol system. The relaxation time (τ) increased with increase in chain length of the alcohols. The result shows that the strength of this molecular interaction depends upon the carbon chain length of the alcohols. Hence the proton donating ability of alcohols is in the order of 1-propanol<1-butanol<1-pentanol
KEYWORDS:
Ethyl formate; Dielectric relaxation; Alcohols
Copy the following to cite this article:
Elangovan S, Mullainathan S. Dielectric Relaxation Studies of Ethyl Formate with Primary Alcohols using Time Domain Reflectometry. Mat.Sci.Res.India;9(1)
|
Copy the following to cite this URL:
Elangovan S, Mullainathan S. Dielectric Relaxation Studies of Ethyl Formate with Primary Alcohols using Time Domain Reflectometry. Mat.Sci.Res.India;9(1). Available from: http://www.materialsciencejournal.org/?p=1181
|
Introduction
The dielectric relaxation studies are one of the useful techniques to elucidate the nature of interactions that exist in solute-solvent component of a system.1-3 Jianhuattu et al have studied the excess enthalpies, excess isobaric heat capacities, densities and speeds of sound of the mixtures ethyl formate + benzene, + ethanol, and + 2,2,2- trifluoroethan-1-ol, at 298.15 K.4 Ethylformate is an ester. P.k.Choi et al have observed rotational isomeric relaxations in methyl and ethyl formates using the Plano-concave ultrasonic resonator method.5 It is used in the lacquer industry as a solvent for cellulose nitrate. It is also used as fumigant and larvacide or tobacco. It is a highly attractive option for bulk disinfestations of unprocessed dried fruit during ware housing. The molecular interaction studies of liquid mixtures with alcohols as one of the components is of particular interest, since alcohols are highly polar and self associated through hydrogen bonding. The carbonyl group (C=O) present in the ethyl formate tends to participate in hydrogen bonding interactions with hydroxyl (OH-) group of alcohols. The present work is an attempt to study the molecular interactions between of ethyl formate with1-Propanol, 1-Butanol and1-pentanol using time domain reflectometry technique at 303K.
Materials and Methods
Ethylformate and alcohols of AR grade were obtained from E-Merck India and used with out further purification. The purity of liquids analysed with the standard physical quality values. The dielectric constant (e’ ) and dielectric loss (e” ) have been measured using X-band microwave frequency oscillator of frequency 9.36 GHz at 303K.The refractive index (µ) of all the solutions has been measured by Abbe’s refractometer. The viscosities were measured with the help of Ostwald’s viscometer. The densities were measured by using 5cc specific gravity bottle.
Higasi’s Method
The dielectric relaxation time (ô) was calculated using Higasi’s method.6 Assuming e e, e’’ and e¥ vary linearly with weight fraction w of the solute. The slopes a0 a, a’’ and a∞ determined from the determined values. We have
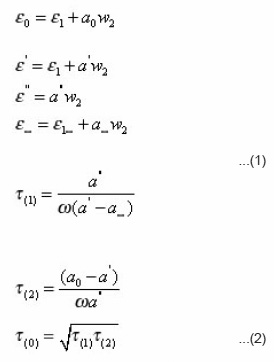
Here (t0) is the mean relaxation time. The free of activation of dielectric relaxation ΔFτ and viscous flow have been calculated using Eyring’s equation7
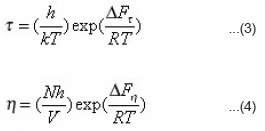
Where h is Planck’s constant. k is Boltzmann constant, N is Avogadro number and V is the molar volume.
Cole-Cole Method
The measured values of e0, e,’ e’’ and e∞ are fitted in a complex plane plot with depress circular arc. The angle made by the diameter d drawn through the centre from the e∞ point and the abscissa axis is equal to πα/2. From the Cole-Cole arc, the relaxation time τ can be found using the equation

Where ω is the angular frequency of the micro wave and α can be obtained from the Cole- Cole plot.
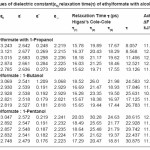
Table 1: Values of dielectric constant(ε0),relaxation time(τ) of ethylformate with alcohols at 303K
Results and Discussion
The dielectric parameters of the selected system have been reported as shown in the table 1. The relaxation time depends up on the size and shape of the rotating molecular entities in the liquid mixture. Here the relaxation time (t) values increase while increase in the concentration of alcohols. This trend may show that the hydrogen bond formation between the C=O group of ethyl formate and the O-H group of the alcohol. Similar conclusions have been reported by Dharmalingam revealed that the viscous flow involved both the rotational and translation form of motion. Similar results were reported as earlier 9-12.The result shows that the strength of this molecular interaction depends upon the carbon chain length of the alcohols. Hence the proton donating ability of alcohols is in the order of 1-propanol<1-butanol<1- pentanol.
Conclusion
Dielectric relaxation parameters have been reported in the paper for primary alcohols with ethylformate in various concentrations at 303K.the relaxation time increases with increasing acidity of proton donor complex systems. The systematic change of dielectric parameters with alcohols show that the proton donating ability of alcohols is in the order of 1-propanol<1-butanol<1-pentanol.
Acknowledgements
The authors are thankful to the Department of Physics, Easwari Engineering College, Chennai, Tamil Nadu, India for providing all facilities to complete this study.
References
- Hanna F.F, Saad. A.L.G Youseef .M.H and Solaiman.A., J Mol Liq., 89: 57 (2000)
CrossRef
- Dharmalingam.K, Ramachnadran .K,Sivagurunathan.P and Kalamse.G.M., Acta Physico-Chimica Sinica., 23: 50 (2007)
- Martinez Jimenez .J.P , Fornies-Marquina.J.M , Digon Rodriguez.D, Otin Lacarra.S.,J Mol Liq., 139: 48 (2008)
- Jianhua Hu , Katsutoshi Tamura and Sachio Murakami., Fluid Phase Equilibria.,131, 197 (1997)
CrossRef
- Choi P. K , Naito.Y, Takagi.K., Chemical Physics Letters.,121: 169 (1985)
CrossRef
- Higasi .K, Koga.Y and Nakamura. M Bull Chem Soc Japan 44: 988 (1971)
- Eyring.F Cernuschi., J.Chem.Phys., 7: 547(1639 )
- Dharmalingam.K., Bull Korean Chem Soc.,27: 2040 (2006)
- Dharmalingam. K, Ramachandran. K,Sivagurunathan. P and Kelamse.P., MainGroup Chemistry., 4: 227 (2005)
- Kalaivani.T, Kumar.S and Krishnan.S., IndianJournal of Chemistry., 43A: 291 (2004)
- Malathi.M, Sabesan.R and Krishnan.S.,Material Science and Engineering., 100B:318 (2003)
- Kalaivani .T, Krishnan.S., Ind.J.Pure andappl.Phs 47: 383 (2009)

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal