Aromatic polyimides have become an important class of polymers that have a wide range of application as high performance materials in the aerospace and industries electronics because of high thermal stability.9-13 Aromatic polyimide having phthalimide pendent group into the polymer are found to possess excellent heat resistance, transparency and enhances the solubility and thermal stability of polymer.14,15
Phthalimide and its derivatives are very important compounds. They used in the synthesis of antimicrobial activity, antiandrogens and other agents for treating tumour necrosis factor. Certain phthalimide derivatives are used as herbicides and for reducing bacterial contamination.16 Copolymers containing the phthalimide derivatives have been used as optical brightening agents.17 N-Substituted phthalimide copolymers may be used as activated drug binding materials.18
In our previous work we described the synthesis, polymerization, copolymerization and exchange reactions of the acrylic and methacrylic esters of N-hydroxyphthalimide and N-hydroxytetrabromophthalimide.19-24 The aim of the present work is to report the synthesis, characterization and determination of reactivity ratios for copolymerizations of 2-(N-phthalimido)ethyl methacrylate (PEMA) with methyl acrylate (MA), ethyl acrylate (EA), butyl acrylate (BA) and styrene (ST).
Experimental
Materials
Methyl acrylate, ethyl acrylate, butyl acrylate and styrene were BDH (England) products. Phthalic anhydride and N,N-dicyclohexylcarbodiimide (DCCI) were from Aldrich Co.. Methacrylic acid and the free radical initiator, azobisisobutyronitril (AIBN), were from E. Merck, Darmstadt. All the solvents were of reagent grade and were purified by distillation before use.
Synthesis
1-Preparation of N-(2-Hydroxyethyl) Phthalimide (NHEP)
Phthalic anhydride (0.1 Mol) was taken in a two-necked flask fitted with a reflux condenser and a nitrogen inlet, and dissolved in 50 ml of DMF, 0.1 mol of ethanolamine was added dropwise to it. The reaction was moderately exothermic hence cooled in an ice bath. The contents were stirred with magnetic stirrer and heated in an oil bath to 130°C in nitrogen atmosphere for 3 h. Excess DMF was removed by vacuum distillation and the contents were poured into water. The precipitated product, NHEP, was filtered off and recrystallised using ethanol as a solvent. The yield was 75% and m.p. was 125-127°C. (Lit.126)25
2-Synthesis 0f 2-(N-Phthalimido)Ethyl Methacrylate (PEMA)
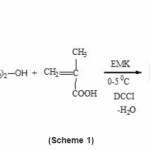
To a cold solution (0–5°C) of 19.1 g (0.1 mol) of N-(2-hydroxyethyl) phthalimide (NHEP) and 8.6 g (0.1mol) of methacrylic acid in ethyl methyl ketone (100 ml) was added 20.6 g of DCCI, with stirring. After stirring for 6 h at room temperature, the precipitated dicyclohexyl urea was removed by filtration and the filtrate was evaporated to dryness in vacuum. 2-(N-Phthalimido)ethyl methacrylate (PEMA), obtained as a white solid, was washed with 0.05% NaOH and recrystallised from ethanol, the yield was 70%, m.p. 75-77°C (Lit.75oC).25
Homopolymerization
Solution (10%) of the monomer in DMF was treated with azobisisobutyronitrile (AIBN) (1mol%). After purging with deoxygenated nitrogen, the reaction mixture was allowed to stand at 60oC for 6h. The polymer was obtained by reprecipitation in methanol and were collected by filtration, washed dried and weighted. The yield was 80%.
Copolymerization Reactions
Copolymers of 2-(N-phthalimido)ethyl methacrylate (PEMA) with methyl acrylate, ethyl acrylate, butyl acrylate and styrene were studied for different mole fractions in the feed. The reaction time was restricted to give conversion less than 10% in order to obtained polymer samples with homogeneous composition.
The copolymers were obtained by solution polymerization method in presence of azobisisobutronitrile (AIBN) as a free radical initiator. Thus the pre-determined amounts of the comonomers were placed in glass tubes, and diluted with dimethyl formamide so that the total monomer composition was about (1.5mol/l). Polymerization was commenced by adding AIBN (1mol%). The tubes were flushed with oxygen-free nitrogen for 10 minutes, capped and thermostated at 650C for 1-3h, depending on the comonomer pairs and composition. All copolymers were obtained by reprecipitation from methanol, except EA reprecipitation from 50% methanol and 50% distilled water and all washed several times, dried and weighed. The composition of copolymers was determined from nitrogen analysis.
Characterization
IR spectra were recorded (KBr) on a Pye-Unicam Sp-883 Perkin Elmer spectrophotometer. 1HNMR spectra were recorded on a Varian Gemini 200 MHz spectrophotometer, Cairo University. The chemical shift (δ) are given downfield relative to tetramethylsilian (TMS) as the internal standard. The elemental analysis were also carried out in Microanalytical center, Cairo University. The antibacterial and antifungal activities were carried out at the Regional Centre for Mycology, and Biotechnology at Microanalyticl Center, Cairo University.
Results and Discussion
In the present investigation, 2-(Nphthalimido) ethyl methacrylate (PEMA) was prepared by the reaction of N-(2-hydroxyethyl) phthalimide (NHEP) with methacrylic acid in the presence of DCCI, according to the following (Scheme1):
The monomer was a crystalline solid, easily soluble in most organic solvents, but sparingly soluble in aliphatic hydrocarbons, such as n-hexane and petroleum ether.
The structure of PEMA monomer was confirmed by IR and 1HNMR spectroscopy.
IR (cm-1): 3100, (aromatic C-H stretching), 3062 (olefinic C-H stretching), 2930 and 2857 (symmetrical and asymmetrical C-H stretching due to CH2 and CH3), 1770 (-CO-OR), 1720 (C=0 stretching due to phthalimido group), 1640 (olefinic C=C stretching).
1HNMR (ppm): 7.95-7.74 (aromatic protons), 6.02 and 5.53 (2H) [olefinic protons], 4.41 (2H) [-CH2-O], 4.01 (2H) [N-CH2] and 1.45 (3H) [Methyl]
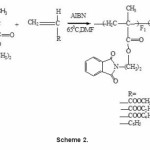
The copolymerization of PEMA with MA, EA, BA and ST were studied and the reaction can be written as shown in (Scheme 2).
In all systems studied, the composition of each copolymer was calculated from its nitrogen content. The structure of the copolymers was confirmed by IR spectroscopy. The IR spectrum of PEMA-ST copolymer, as example, shows peak at 3030 cm-1 is due to the C-H stretching of aromatic protons. Peaks at 2929 and 2858 cm-1 corresponding to the C-H stretching of methyl and methylene groups. The strong absorption peaks at 1770 and 1750 cm-1 are due to the ester carbonyl stretching and that at 1732 cm-1 is due to the carbonyl stretching of the phthalimido group and a strong band at 760 cm-1 due to monosubstituted benzene ring.
For the industrial preparation of any copolymers, knowledge of reactivity ratio is very essential. The reactivity ratios of PEMA with MA, EA, BA and ST were evaluated from the monomer feed ratios and the copolymer composition.
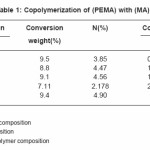
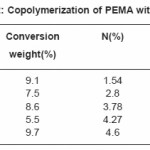
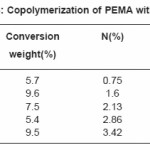
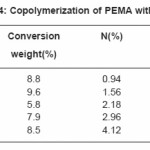
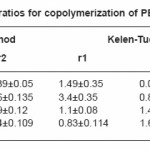
Tables (1-4) illustrate the analytical data for copolymerization reactions of PEMA with MA, EA, BA and ST. From the values of feed and copolymer composition, the monomer reactivity ratios (r1 and r2) for each system were evaluated using Fineman –Ross27 and Kelen–Tudos28 methods and the standard deviations of the results were calculated by regression analysis as given in Table (5). Fig. (1) shows the Kelen–Tudos28 plots for the four systems studied. The values of r1 and r2 obtained by Fineman–Ross and Kelen– Tudos are almost identical. The r1r2 value for PEMA-MA (0.15) indicates that the copolymer should have a random distribution of the monomer units, while for the PEMA-EA, PEMA-BA and PEMA-ST systems the r1r2 values (2.5, 1.5 and 1.32) respectively, illustrate a low tendency of monomers to alternated, and the copolymer should be composed mainly of small sequences of monomeric units of the same type. The composition curves of the four binary copolymerization reactions studied are illustrated in Fig. (2) which indicates that all studied systems gave no azeotropic compositions.
The Q and e values were calculated by using the Alfrey-Price equations29
e1 = e2 ± (-In r1r2)1/2,
Q1 =(Q2/ r2 )exp[-e2(e2 – e1) ]
The Q and e values which represent the extent of resonance stabilization and the polarity of the double bond, respectively, in a monomer and its radical are extensively tabulated by Young30 from earlier copolymerization data. Thus, the average Q and e values for PEMA were obtained by using the monomer reactivity ratios determined for the copolymer systems and using the Q and e values for the vinyl monomers. The average Q and e values for NPEMA were found to be Q =1.4 and e = 0.51 and are in agreement with those reported in the literature30 for the esters of methacrylic acid.
Scheme 1:
Scheme 2:
Table 1: Copolymerization of (PEMA) with (MA)
Table 2: Copolymerization of PEMA with (EA)
Table 3: Copolymerization of PEMA with (BA)
Table 4: Copolymerization of PEMA with (ST)
Table 5: Monomer reactivity ratios for copolymerization of PEMA with MA, EA, BA and ST
Figure 1: Kelen-Tudos plots for copolymerizations of PEMA with: (a)MA, (b)EA, (
c)BA and (d) ST :ζ=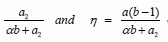
where a and b are the molar ratios (M1/M2) of the comonomer in the feed and copolymer, respectively, and α =
Antimicrobial Activity
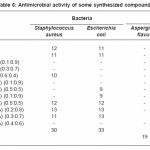
The antimicrobial activity of the synthesized compounds was determined in vitro using the hole plate and filter paper method24 against a variety of bacteria and fungi. Comparative studies of our prepared compounds and standard drug were also carried out . The tested compounds were dissolved in DMSO, different concentration have been chosen (125, 250 and 500 µg/ml). The inhibition zones of microbial growth produced by different compounds were measured in millimeters at the end of an incubation period of 48h at 28oC. DMSO alone showed no inhibition zone. The gram +ve bacteria were Staphylococcus aureus; the gram –ve bacteria were Escherichia coli and the fungi were Aspergillus flavus and Candida albicans. Tetracyclin and Amphotericin B were used as standard for antibacterial and antifungal activity, respectively. The results are illustrated in (Table 6).
Table 6: Antimicrobial activity of some synthesized compounds
Figure 2: Composition curves for copolymerization of PEMA with: (a)MA, (b)EA, (c)BA and (d)ST
Conclusion
Copolymers from PEMA with MA, EA, BA and ST with different compositions were easily synthesized in DMF by free radical polymerization. The monomer reactivity ratios (r1 and r2) for each system were evaluated using Fineman –Ross and Kelen–Tudos methods and the values obtained are in good agreement. The r1r2 value for PEMA-MA (0.15) indicates that the copolymer should have a random distribution of the monomer units, while for the PEMA-EA, PEMA-BA and PEMA-ST systems the r1r2 values (2.5, 1.5 and 1.32) respectively illustrate a low tendency of monomers to alternated, and the copolymer should be composed mainly of small sequences of monomeric units of the same type. The composition curves indicates that all studied systems gave no azeotropic compositions. The average Q and e values for PEMA are in agreement with those reported in the literature for the esters of methacrylic acid. (0.15) indicates that the copolymer should have a random distribution of the monomer units, while for the PEMA-EA, PEMA-BA and PEMA-ST systems the r1r2 values (2.5, 1.5 and 1.32) respectively illustrate a low tendency of monomers to alternated, and the copolymer should be composed mainly of small sequences of monomeric units of the same type. The composition curves indicates that all studied systems gave no azeotropic compositions. The average Q and e values for PEMA are in agreement with those reported in the literature for the esters of methacrylic acid.
References
- Liu, G.; Zhang, L.; Wang, Y.; Zhao, P. J Appl Polym Sci 114: 3939 (2009).
CrossRef
- Saby-Dubreuil, A. C.; Guerrier, B.; Allain, C.Polymer, 42: 1383 (2001).
CrossRef
- Erol, I.; Sen, O.; Dedelioglu, A.; Cifci, C. J Appl Polym Sci, 114: 3351 (2009).
CrossRef
- Habibi, A.; Farahani, E.V.; Semsarzadeh, M.A.; Sadaghiani, K. Polym. Int, 52: 1434 (2003).
CrossRef
- Hou, C.; Ji, C.; Ying, L. J Appl Polym Sci 103: 3920 (2007).
CrossRef
- Miller, A.; Szafko, J.; Turska, E. J. Polym. Sci., 15: 51 (1977).
- Bajaj, P.; Sen, K.; Hajir, B.S. J Appl Polym Sci, 59: 1539 (1996).
CrossRef
- Soykan, C.; Coskun, M.; Ahmedzade, M.Polym. Int., 49: 479 (2000).
CrossRef
- Cassidy, P. E.; Marcel &Dekker, New York, 94-103 (1980).
- Yang, H.H.; Wiley, New York, 63-98 (1989).
- Mittal K.L.; New York, I & II, 63-67 (1984).
- Ye, Y.S.; Yen Y.C.; Chen W.Y.; Cheng C.C.; Chang F.C.; J Polym Sci Part A: Polym Chem, 46: 6296 (2008).
CrossRef
- Rubehn, B.; Stieglitz T. Biomaterials, 31: 3449 (2010).
CrossRef
- Payene, H.F.; New York: Wiley; Biomaterials,1: 27 (1964).
- Marton, O.R.; New York: Reinhold, “Technology of paints, varnishes and lac- quers” 32: 32 (1968).
- Krishnakumar, A.; Balachandran, V.B.; Chithambarathanu, T.; Spectrochimica Acta Part A., 62: 918 (2005).
CrossRef
- Constantinova, T.N.; Garbechev, I.K., Polym Int 43: 39 (1998).
CrossRef
- Ismail, M; Veena, V.; Animesh, K.R.; J Appl Polym Sci, 68: 217 (1998).
CrossRef
- Shaaban, A. F.; Arief, M. M. H.; Khalil, A. A.; Messiha, N. N., Acta Polym 39: 145 (1988).
CrossRef
- Shaaban, A. F.; Khalil, A.A.; Messiha, N. N. J Appl Polym Sci, 37: 2051 (1989).
CrossRef
- Shaaban, A. F.; Khalil, A. A.; Messiha, N. N. Acta Polym, 40: 445 (1989).
CrossRef
- Mahmoud, A. A.; Shaaban, A. F.; Khalil, A. A.; Messiha, N. N. Die Angew Makromol Chem, 198: 31 (1992).
CrossRef
- Khalil, A. A.; Moustafa, H. Y.; Arief, M. M. H. Eur Polym J 1996, 32, 647.
CrossRef
- Khalil, A. A. J Appl Polym Sci, 99: 2258 (2006).
CrossRef
- Jayakumar, R; Balaji, R.; Nanjundan, S. Eur Polym J, 36: 1659 (2000).
CrossRef
- Lefiert, C.; Li. H.; Chidburee, S.; Hampson, S.; Workman, S.; Digel, D.; Epton, H.A.S.; Harbour, A. J. Appl. Bacteriol., 78: 97 (1995).
- Fineman, M.; Ross, S. D. J. Polym. Sci., 5: 258 (1950).
CrossRef
- Kelen, T.; Tudos, F. J. Macromol. Sci. Chem.,A9 (1975).
- Alfrey, T. Jr.; Price, C. J; J. Polym. Sci., 2: 101 (1947).
CrossRef
- Young, L. J. Polymer Handbook 2nd ed. J. Brandrup and E. H. Immergut, Eds., New York, Wiley interscience pp.II 105-II.404 (1975).
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal