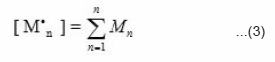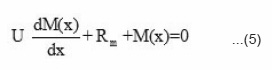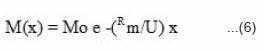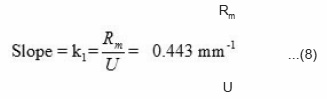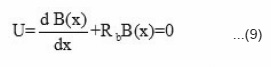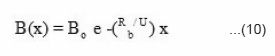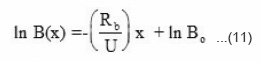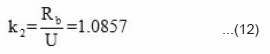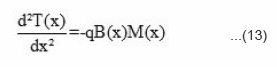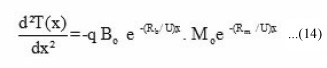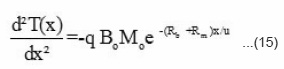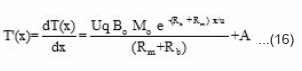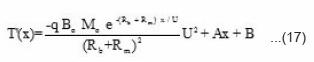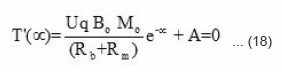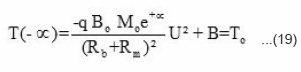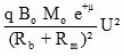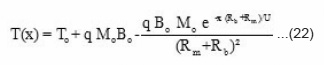Introduction
The studies of frontal polymerization began in 1972 in the former Soviet Union1-2. They studied frontal polymerization of methyl methacrylate and discussed how various factors such as the initial concentration and temperature, ambient pressure, the nature of the initiator, etc. affected the characteristics of the propagating wave, its velocity and the composition of the polymer. Later on, several other groups confirmed these findings3-4 and thereafter extensive work is being done on frontal polymerization.5-7
Non-linear phenomena in polymerization reactions are interesting because of a rich variety of non-linear behaviour such as oscillations, convection, chaos and propagating wave fronts. Teymour and Toy have reported a wide range of dynamical behaviour : oscillations in temperature, oscillation in extent of polymerization and period doubling route to chaotic oscillations.7-8 Periodic behaviour in emulsion polymerization have been reported in a continuously stirred tank reactor.9
Pojman have reported traveling wave front in methacrylic acid polymerizations.10-11 Srivastava et al. have investigated banded structure in methacrylic acid / methanol / benzoyl peroxide / N,N-dimethyl aniline polymerization reaction in a glass tube.6,12 A mathematical modeling of propagating polymerization fronts driven by the exothermic polymerization reaction and the transport of heat from the polymerized product have been reported.13 The use of polymerization processes based on this mode of polymerization has many applications including rapid curing of polymers without external heating, unicuring of thick samples, solvent less preparation of some polymers, and filling / sealing structures having cavities of arbitrary shape without having to heat the structure externally.14-16
Experimental
Benzoyl peroxide and hydroqunione (CDH), N,N-dimethyl aniline (BDH) of analytical grade were used without further purification. Acrylic acid (SISCO) was double distilled under reduced pressure to remove inhibitor. The solutions of benzoyl peroxide and hydroquinone were prepared separately in acrylic acid. Freshly prepared solutions were used each day. The solution of benzoyl peroxide in acrylic acid was taken in a glass tube having an inner diameter of 13mm. The volume of solution in test tube was kept constant 9.0 ml and the length of the solution in test tube was 7.0 cm. The reaction was started by adding N, N-dimethyl aniline from the top of the tube by a micropipette. After addition of N, N-dimethyl aniline a solid polymer mass nucleate at the top of the tube slution and it takes few minutes to convert into the form of a sharp wave front. This wave front moves downward with a constant velocity. All measurements were performed with descending fronts. The velocity of the front was measured with a cathetometer. All the experiments were performed in an air thermostat. The temperature profile was measured by using a miniature thermocouple and an Orion digital thermometer attached to a strip chart recorder. The temperature versus time data was converted to a temperature versus front position using measured front velocity.
Results and Discussions
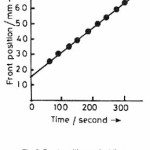
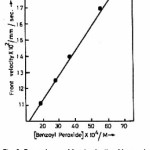
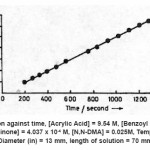
Frontal polymerization of acrylic acid with benzoyl peroxide was started by adding N, N-dimethyl aniline [0.05 ml of 7.89 M] at the top of the solution in the test tube containing acrylic acid , benzoyl peroxide [1.83 x 10-3 M], hydroquinone [2.02 x 10-4 M]. A solid polymer mass nucleates at the top of the solution and it takes a few minutes to convert into the form of a sharp wave front. This wave front moves downward with a constant velocity. A typical movement of front is shown in Fig. 1 and the corresponding position of front with respect to time is shown in Fig. 2. The dependence of front velocity on initial concentration of benzoyl peroxide is shown in Fig. 3. It is clear from the figure that front velocity increases on increasing the benzoyl peroxide concentration. The front movement is slowed down when hydroquinone is added into the system which would be evident from a comparison of Fig. 4 with Fig. 2.
When N, N-dimethyl aniline is added from the top of solution benzoyl peroxide breaks into radicals. This decomposition occurs at room temperature, and it can be represented as
If f is the fraction of free radical actually consumed in the reaction, the rate of initiation is given by
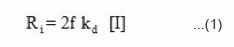
This free radical attacks the double bond of acrylic acid monomer to produce a new radical consisting of the initiator fragment and one monomer unit. Subsequently, the propagation reaction represented below follows
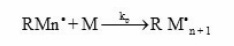
and the rate expression for propagation may be given as

where [ M ] is the monomer concentration and [M·n ] represents the concentration of polymer radical of all sorts varying in the degree of polymerization 1 to n, and kp is the rate constant for propagation.
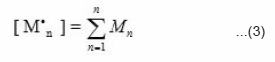
The chain reaction ends with termination.

where [ P • ] is the total concentration of polymer radicals, kt is the rate constant for chain termination. P n denotes the polymer radical containing n monomer unit, and P m denotes the polymer radical containing m monomer units. The N, N-dimethyl aniline reacts with benozyl peroxide and forms free radicals. These radicals react with monomer and form a front. This front moves downward with definite velocity liberating heat which acts as a driving force for the front. If Mo is the initial concentration of monomer, Rm is the reaction rate of monomer, and U is the front propagation velocity. Then the kinetics of disappearance of monomer M(x) with front position can be expressed as
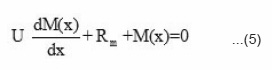
the solution of this equation takes the form
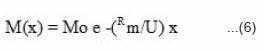
Expressing equation (6) in logarithmic form
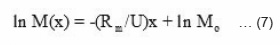
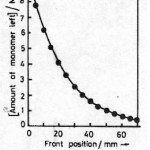
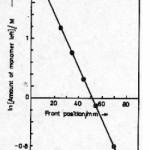
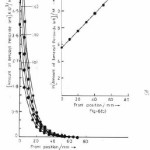
Amount of monomer left against front position was experimentally determined by measuring the mass of monomer contained in unit distance multiplied by distance traveled by front and is shown in Fig. 5(a). The logarithmic plot of this figure 5(a) is shown in Fig. 5(b). The rate constant R1 and propagation velocity U was determined by comparing the experimental Fig. 5(a) & 5(b) with equation 7.
The rate constant k1 of the reaction is equal to the slope of the line in Fig. 5(b).
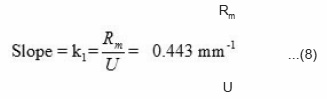
The propagation velocity U can be determined experimentally from the slope of the curve shown in Fig. 2. This comes out to be 0.167 mm/second. Putting the value of U in equation (8) the reaction rate of monomer concentration Rm. can be calculated as
Rm = 0.443U – 0.074 mole/mm
Propagation velocity U for hydroquinone (Fig. 4) = 16.55 mm/second, the rate constant will remain same as in eq (8), but reaction rate of monomer consumption will change, Rm = 0.437 × 16.55 = 7.332 mole/mm.
Decrease in benzoyl peroxide concentration with respect to front position can be expressed by a differential equation (9) where B(x) is the amount of benzoyl peroxide left at distance x from the top of the solution and Rb is the reaction rate of benzoyl peroxide.
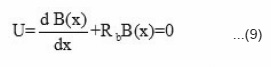
and solution of this equation is
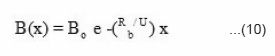
Taking ln of equation (10) we get
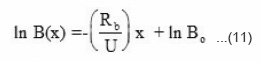
The amount of benzoyl peroxide left against front position was determined experimentally by measuring the mass of benzoyl peroxide contained in unit distance multiplied by distance traveled by front and is shown in Fig. 6(a) for different concentrations of benzoyl peroxides. The logarithmic plot of amount of monomer left against front position is shown in Fig. 6(b). The rate constant k2 and propagation velocity U was determined the experimental Fig. 6(a) and 6(b) with equation (11). The slope of this plot gives the rate constant of this reaction k2 and is equal to
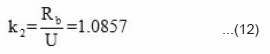
and intercept will give Bo = 0.0039 M.
On substituting the propagation velocity U= 0.16667, reaction rate for benzoyl peroxide system can be calculated as
Rb = 1.0857 × 0.16667 = 0.18095 mol / mm.
and for hydroquinone system propagation velocity, U = 16.55
and
Rb = 1.0857 X 16.66 = 18.088 mol / mm
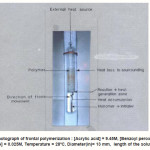
Figure 1 (a): Photograph of frontal polymerization : [Acrylic acid] = 9.45M, [Benzoyl peroxide] = 0.003 M, [N,N-DMA] = 0.025M, Temperature = 28oC, Diameter(in)= 13 mm, length of the solution 70 mm
Figure 2: Front position against time, concentration and conditions are same as in figure 1
Figure 3: Dependence of front velocity of benzoyl peroxide concentration, other concentrations and conditions are same as in figure 1
Figure 4: Front position against time, [Acrylic Acid] = 9.54 M, [Benzoyl peroxide] = 0.003M, [Hydroquinone] = 4.037 x 10-4 M, [N,N-DMA] = 0.025M, Temp. = 28oC, Diameter (in) = 13 mm, length of solution = 70 mm
Figure 5(a): Plot of amount of monomer left against front position, the composition and conditions are same as in figure 1
Figure 5(b): Logarithm plot of amount of monomer left against front position of figure 5(a)
The frontal polymerization is an exothermic and the heat evolved depends mainly on the initiator concentration [BP] and on initial reaction temperature. The role of N, N, -DMA is only to start the reaction and nucleation and does not contribute to rise in temperature. The variation of reaction temperature T(x) with front position can be expressed by the differential equation
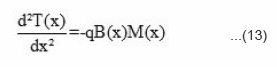
where q = H / C ρ, H = change in the reaction enthalpy, C = specific heat of reaction mixture, and ρ = density of the reaction mixture. B(x) = benzoyl peroxide concentration left at the distance x and M(x) = amount of monomer left at distance x. On substituting the values of M(x) and B(x) from equation (6) and (10) respectively, we get
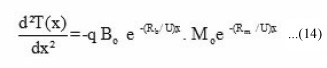
On simplification we have
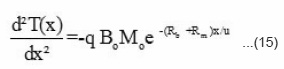
The boundary conditions for the system (15) can be taken as
when, x = +∝, T'(x) = 0
when, x = -∝, T(x) = To
Further, we get
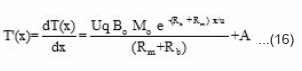
and
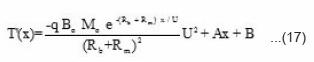
where A and B are constants, the value of constant A can be calculated by applying the initial condition x = ∝.
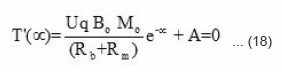
Since e– ∝ = 0, therefore, A = 0.
Further, the value of constant B can be calculated by taking the initial condition x = – ∝.
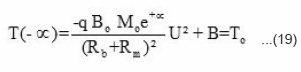
If we assume that q Bo Mo is a suitable large value taken from the domain of
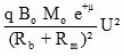
we get
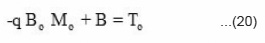
or
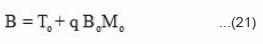
On substituting these values in eq (17) T(x) can be expressed as
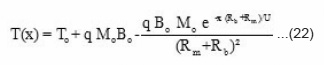
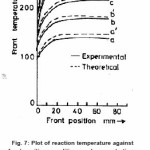
from equation (22), the reaction temperature T(x) has been calculated at different initial concentration of benzoyl peroxide and plotted against front position in Fig. 7. The experimental results (a solid line) measured by. thermocouple is also shown in Fig. 7. It is clear from the figure that reaction temperature increases sharply and attains a maximum saturation value whatever be the position of front. Further, on increasing the benzoyl perozxide concentration reaction temperature increases.
Figure 6: (a) Plot of amount of benzoyl peroxide left against front position, condition and concentrations are same as figure 1 except benozyl peroxide. (a) 0.003M, (b) 0.004M, (c) 0.005M, (d) 0.006M, (e) 0.007M Fig. 6(b): Logarithm plot of amount of benzoyl peroxide left against the front position for, [benzoyl peroxide] = 0.004M
Figure 7: Plot of reaction temperature against front position, conditions and concentration are same as in figure 1, except the change in benzoyl peroxide concentration (a) 0.003M, (b) 0.004M, (c) 0.005M, (d) 0.006M, (e) 0.007M, q = 4000, B0 = 0.003M, M0 = 9.54M, Rm = 0.037 M, Rb = 0.09, T0 = 30oC, U = 0.085,where, a, b, c, d, e (experimental) (________) and a’, b’, c’, d’, e’ (theoretical) (—–)
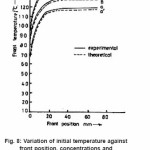
Figure 8: Variation of initial temperature against front position, concentrations and compositions are same as figure 1, except the initial temperature (a) = 5oC, (b) = 15oC and (c) = 300C, q = 4000, B0 = 0.003M, M0 = 9.54M, Rm = 0.037 M, Rb = 0.09, T0 = 30oC, U = 0.085, where, a, b, c (experimental) (_____) and a’, b’, c’ (theoretical) (—-)
The reaction temperature is also very sensitive to initial reaction temperature the dependence of reaction temperature with respect to front position is calculated by equation (22) at different initial temperature is shown in and corresponding experimental value is shown in Fig. 8 solid lines. It is clear from the figure that reaction temperature increases sharply with front position and attains a maximum saturation value. Further, this rise in temperature increases with increasing the initial reaction temperature. The maximum rise in temperature is 143oC at 30o C initial reaction temperature. The rate of chain propagation Rp and the front velocity (U) mainly depends upon the rate of consumption of monomer (Rm) and rate of consumption (Rb) of benzoyl peroxide. It is not unreasonable to assume Rp = (Rm + Rb) , and can be determined by the knowledge of Rm and Rb. Which also explains the rise in temperature with respect to benzoyl peroxide concentrations and initial reaction temperature.
References
- Cheabilo, N.M., Khvilivitsky, R. Ya. and Enikolopyan N.S, Dokl. Phys. Chem. 204:512-513 (1972).
- Chechilo, N.M. and Enikolopyan, N.S., Dokl.Phy. Chem. 230: 840-843 (1976).
- Volpert, Vit. A. and Volpert V A., Eur. J. Appl.Math. 5: 201-215 (1994).
- Pojman, J.A., Graven R., Khan, A. and West., W., J. Phys. Chem. 96: 7466-7472(1992).
CrossRef
- Pojman, J.A., Nagy, I.P. and Salter, C., J. Am.Chem. Soc. 115: 11044-11045 (1993).
CrossRef
- Teymour, F and Roy, W.H., Chem. Eng. Sci.47: 4121 (1992).
CrossRef
- Teymour, F and Roy, W.H., Chem. Eng. Sci.47: 4133 (1992).
CrossRef
- Schork, F.J. and Roy, W.H., J. Appl. Poly. Sci.(Chem.) 34: 1259 (1987).
CrossRef
- Pojman, J.A., J. Am. Chem. Soc. 113: 6284(1991)
CrossRef
- Pojman, J.A., Curtis, G. and Ilyashenkov, M.,J. Am. Chem. Soc. 118: 3783 (1996).
CrossRef
- Srivastava, P.K., Majhi, S.S., Rastogi, R.P.and Hanazaki, I., Chemistry Letters, 1251(1998).
CrossRef
- Srivastava, P.K. and S.S. Majhi, J. Indian Chemical Soc., 80: 559-561 (2003).
- P.M. Goldfeder and V.A. Volpert, V.M.Ilyashenko, A.M. Khan, J.A. Pojman and S.E. Solovyov. J. Phys. Chem. B, 101: 3474(1997).
CrossRef
- Jun Tu, Li Chen, Yuan Fang, Caifeng Wang,Su Chen, Journal of Polymer Science PartA-polymer Chemistry, 48(4): 823-831 (2010).
CrossRef
- Qiao Feng, Fang Li, Qing-Zhi Yan, Ying-Chun Zhu, Chang-Chun Ge , Colloid and Polymer Science, 288(8): 915-921 (2010).
CrossRef
- Belk M, Kostarev K.G, Volpert V and Yudina T, J. Phys. Chem. B, 107: 10292 (2003).
CrossRef
Views: 405
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal