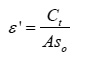Dielectric, Thermal and FTIR Studies of Cobalt Doped Magnesium Hydrogen Maleate Hexahydrate Single Crystals
B. Rajagopal1*, M. V. Ramana2 and A. V. Sarma3
1Department of Physics, Kavitha Degree College, Khammam - 507002, India
2Department of Physics, S.R & B.G.N.R. Degree College, Khammam - 507002, India.
3Department of Physics, College of Science and Technology, Andhra University, Visakhapatnam - 5300003, India.
DOI : http://dx.doi.org/10.13005/msri/080112
Article Publishing History
Article Received on : 25 Apr 2011
Article Accepted on : 29 May 2011
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Present work reports the dielectric, thermal and FTIR studies of Co2+: MHMH single crystal for the first time. The influence of addition of transition metal ions on the dielectric properties, like dielectric constant, loss tangent,a.c. conductivity and activation energy of these crystals was discussed. FTIR studies confirm the presence of carboxylate ions. The melting point of the sample and final product after decomposition are studied using TG/DTA.
KEYWORDS:
Dielectric constant; Loss tangent; Thermal; FTIR
Copy the following to cite this article:
Rajagopal B, Ramana M. V, Sarma A. V. Dielectric, Thermal and FTIR Studies of Cobalt Doped Magnesium Hydrogen Maleate Hexahydrate Single Crystals. Mat.Sci.Res.India;8(1)
|
Copy the following to cite this URL:
Rajagopal B, Ramana M. V, Sarma A. V. Dielectric, Thermal and FTIR Studies of Cobalt Doped Magnesium Hydrogen Maleate Hexahydrate Single Crystals. Mat.Sci.Res.India;8(1). Available from: http://www.materialsciencejournal.org/?p=2498
|
Introduction
Present work reports the dielectric, thermal and FTIR studies of Co2+: MHMH single crystal for the first time. The influence of addition of transition metal ions on the dielectric properties, like dielectric constant, loss tangent,a.c. conductivity and activation energy of these crystals was discussed. FTIR studies confirm the presence of carboxylate ions. The melting point of the sample and final product after decomposition are studied using TG/DTA.
Metal derivatives of unsaturated dicarboxylic acids constitute an abundant group of compounds that are of interest from the viewpoint of both coordination and macromolecular chemistry. The maleates are of practical importance because of their use as coatings with specific properties, efficient catalysts and are also of medicinal significance,1 M.P. Gupta et al.,2 and F. Vanhouteghem et al3 worked on the structure of MHMH crystal. The synthesis and crystal structures on alkali metal maleates were studied recently by Michel Fleck et al.4 The IR spectra of transition metal (VO2+ and Co2+) doped zinc hydrogen maleate tetrahydrate crystals were reported by Rao et al.5
The absorption IR spectra of Cu2+ doped magnesium hydrogen maleate hexahydrate single crystals were studied by S.N. Rao et al.6 The FTIR spectra of Mn2+ doped cobalt maleate tetrahydrate crystals were studied by N.O. Gopal et al.,.7 Thermally stimulated depolarization and thermal studies were carried out on pure and Cu2+ doped potassium hydrogen maleate single crystals by A.D Reddy et al.8 The TG and DTA for maleic acid complexes of manganese, cobalt, nickel, copper and zinc were reported by J.R. Allan et al.,.9 The dielectric, thermal and FTIR studies on the present sample are reported for the first time.
Material and Methods
Cobalt doped magnesium hydrogen maleate hexahydrate (hereafter Co2+: MHMH) single crystal were grown from the aqueous solution containing magnesium carbonate and maleic acid by slow evaporation method at room temperature. Co2+ ions were introduced to an extent of 0.1 mol %, by the addition of solution of their respective sulphates, using AnalaR grade reagents. The crystals are platy and coloured. Good quality Co2+: MHMH crystals were grown from aqueous solution within a period of two weeks. Recrystallisation yielded large size crystals with high transparency. A.C. electrical characteristics of Co2+: MHMH single crystals were measured using Multifrequency Hioki 3522-50 LCR Hi-Tester. Using the LCR meter the data of fourteen parameters such as |Z|, |Y|, θ, Rp(DCR), Rs (ESR, DCR), G, X, B, Lp, Ls, Cp, Cs, D(tan δ) and Q can be measured at different temperatures 308K, 323K, 343K, 363K and 383K respectively. The presence of functional groups in Co2+: MHMH crystal was studied using FTIR spectra recorded using Perkin Elmer FTIR spectrum one spectrophotometer in the range 450 cm-1 to 4000 cm-1 in KBr medium. In order to know the thermal behaviour of the sample, it was subjected to thermogravimetric analysis. The thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were carried out using Netzsch STA 409 C/ CD thermal analyzer at a heating rate of 10 °C/min in the nitrogen atmosphere.
Results and Discussion
Dielectric studies
The dielectric constant of the Co2+: MHMH crystal was calculated by using the relation,
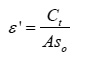
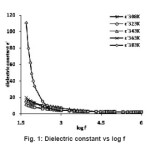
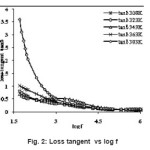
where C is capacitance, t is the thickness, εo the free space permittivity and A the area of the sample. The dielectric constant of the sample under investigation at different temperatures 308K, 323K, 343K, 363K and 383K over 50 Hz to 1 MHz were presented in fig 1. It was observed from the fig 1 that the dielectric constant (ε’) has higher values at lower frequencies and decreases with increasing frequency. The dielectric constant is found to depend on temperature. The dielectric constants of the sample are observed to be 4.34, 4.35, 4.68, 5.10 and 7.16 at temperatures 308K, 323K, 343K, 363K and 383K respectively at 1 kHz frequency. The frequency dependence of dielectric constant å‘ at different temperature shows that at high frequencies the dielectric constant values are low and reaches constant value. As frequency decreases the dielectric constant value becomes more temperature sensitive. The dielectric constant is found to be 11.77 at 50 Hz and decreases to 2.18 at 1 MHz at 308K while it was 110.65 at 50 Hz and it decreases to 2.17 at 1 MHz at 383K. In the lower frequency region high value of dielectric constant can be assigned to the presence of interfacial (space charge) polarization mechanism10-11 and the low value at higher frequencies may be due to the loss of significance of these polarizations gradually. It was observed from the fig 2 that the loss tangent has higher values at lower frequencies and decreases with increasing frequency. The loss tangent is found to depend on temperature and frequency. The variation in the value of loss tangent is low in the frequency range 3.5 kHz – 1 MHz at all temperatures. At low frequencies the dipoles can easily switch alignment with the changing field. As the frequency increases the dipoles are less able to rotate and maintain phase with the applied field, thus they reduce their contribution to the polarization field. The low loss tangent at high frequency is generally expected in the samples with good optical quality. From these results, it can be concluded that the crystal under present study possess good optical quality and defect number is very low.12 It is observed from the fig 3 that the a.c. conductivity increases with increasing frequency according to the relation
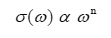
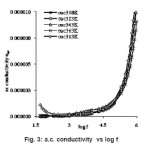
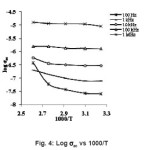
where ω is the angular frequency and the value of ‘n’ is frequency exponent depends on the temperature and frequency. At low frequencies a.c. conductivity was observed to be independent of the frequency. This can be assigned to the domination of percolative behaviour.13 The a.c. conductivity of the sample are observed to be 7.93×10-8, 7.9×10-8, 20.62×10-8, 10.19×10-8 and 14.14×10-8 (Ω-1m-1) at temperatures 308K, 323K, 343K, 363K and 383K respectively at 1 kHz frequency. At high frequencies, the a.c. conductivity is almost proportional to the frequency. This is the characteristic of the intrinsic hopping conductivity. The a.c. conductivity of the sample increases with increase in temperature and the increased a.c. conductivity at high temperatures and high frequency could be due to the reduction in the space charge polarization which is in good agreement with the reported literature.14 It is observed that the electrical conduction the sample is low at low temperature. This behaviour can be assigned to the trapping of some carriers at defect sites in the crystal. The activation energy of conduction E is calculated from the slopes of the plot log (σac) against 1000/T presented in fig 4. It is observed that the slopes are decreasing with increasing temperature. The activation energy is 1.39 eV for 100 Hz frequency between the temperatures 363K to 383K.
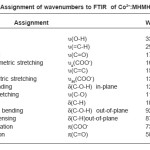
Table 1: Assignment of wavenumbers to FTIR of Co2+:MHMH crystal
Figure 1: Dielectric constant vs log f
Figure 2: Loss tangent vs log f
Figure 3: a.c. conductivity vs log f
Figure 4: Log σac vs 1000/T
Thermal Studies
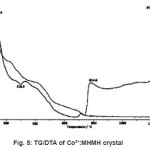
The thermogravimetric analysis of the sample was carried out between 296K and 1673K in nitrogen atmosphere. The thermogram of TG/DTA of the sample is shown in fig 5. In the TG/DTA shows an endothermic peak at 411.4K which can be attributed to the melting point of the sample. The sample starts loosing water at 381.4K. The melting point of the sample was separately determined by capillary method and it was found to be 403K. It is close to the observed value which is presented in DTA (fig 4) of the sample. Heating the sample above melting point results in the formation of volatile substances CO and CO2 and rest of water molecule. The TGA trace shows origin of major weight loss immediately after loss of water of crystallization. Therefore loss of water of crystallization, melting point and decomposition are expected to occur in sequential manner. An exothermic peak is observed at 591.3K. Prolonged heating upto 1087.6K does not produce any significant endothermic or exothermic peaks in DTA curves. The loss of weight on heating the sample started almost in a continuous manner attaining constancy in weight at different temperatures. The weight loss of the sample from the TGA suggests that the final product after decomposition leaves a residue MgO at 1087.6K whose molecular weight (39.77) is approximately equal to its theoretical calculated value (40.30).
Figure 5: TG/DTA of Co2+:MHMH crystal
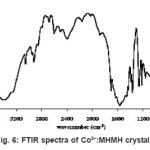
Figure 6: FTIR spectra of Co2+:MHMH crystal
FTIR Studies
The characteristic assignment of wavenumbers of the FTIR spectrum of cobalt doped MHMH crystal is shown in figure 6. The assignment of stretching and bending wavenumbers of the functional groups of the present sample for investigation are presented in table 1.
The assignments are in good agreement with that of the absorptions obtained in carboxylic compounds in the literature5,15-16. The O-H (hydroxyl) stretching appears at 3396 and 3057 cm-1. The alkenyl (C-H) stretching is identified at 2925 cm-1. The bands observed at 1571 and 1395 cm-1 have been assigned to alkene (C=C) stretching and symmetric carboxylate COO- stretching vibrations and that observed at 1672 cm-1 is due to the acid stretch. A weak C=O stretching is assigned at 1706 cm-1. The in-plane bending and out-of-plane bending of C-O-H are observed at 1222 and 929 cm-1 respectively in the present sample. The bending of C-H is present at 1086 cm-1 in the present sample. The C-H out-of-plane, carboxylate anion (COO¯) and carbonyl (C=O) deformations are assigned 871, 731 and 585 cm-1 respectively.
The two carboxylate groups on each maleate anion are coordinated to the magnesium metal. According to resonance, it is important to realize that the carboxylate anion does not show the normal carbonyl and normal C-O single bond stretches in the present investigations of MHMH crystal.
Conclusions
Dielectric properties, thermal studies and FTIR analysis of Co2+: MHMH single crystals were reported for the first time. Dielectric properties suggest that these crystals possess enhanced optical quality with lesser defects. From the TG/DTA plot the final product of the present sample after decomposition at 1073K leaves a residue MgO. In the FTIR studies, the presence of carboxylate ion was confirmed. However, the carboxylate anion does not show the normal carbonyl and normal C-O single bond stretching in the present investigations.
Acknowledgements
The authors would like to thank Prof. S. Jerome Das for recording the dielectric data on the crystals under study.
References
- N. P. Porollo, Z. G. Aliev, G. I. Dzhardimalieva, I. N. Ivleva, I. E. Uflyand, A. D. Pomogailo and N. S. Vanesyan, Russian Chemical Bulletin 46: 362-370 (1997).
CrossRef
- M.P. Gupta, C.Van Alsenoy and A.T.H. Lenstra, Acta Cryst, C40: 1520 (1984).
- F. Vanhouteghem, A.T.H. Lenstra and P. Schweiss. Acta cryst., B43: 523 (1987).
- Michel Fleck and l. Bohaty, Zeitschrift Für Natur for Schung, Graphical abstracts, 64b: 517 (2009).
- S.N. Rao, K. Ramesh and Y.P. Reddy, Solid State Communications, 70: 709-712 (1989).
CrossRef
- S.N. Rao, Y.P. Reddy and P.S. Rao, Solid State Communication, 78: 1025 (1991).
CrossRef
- N.O. Gopal, K.V. Narasimhulu and J. Lakshmana Rao, J Phy and Chem of Solids,63: 295 (2002).
CrossRef
- A.D. Reddy, S.G. Satyanarayana and G.S. Sastry, Pramana, 22: 49 (1984).
CrossRef
- J.R. Allan, G.M. Baillie, J.G. Bonner, D.L. Gerrard and S. Hoey, Thermochimica Acta,143: 283 (1989).
CrossRef
- F. Yogam, I. Vetha Potheher, A. Cyrac Peter, S. Tamilselvan, A. LeoRajesh, M. Vimalan and P. Sagayaraj, Advances in Applied Science Research, 2(1): 261-268 (2011).
- S. Tamilselvan, X. Helan Flora, A. Cyrac Peter, M. Gulam Mohamed, C.K. Mahadevan, M. Vimalan and J. Madhavan, Archives of Applied Science Research, 3(1): 235-240 (2011).
- Balarew C., Dehlew R., J. Solid State Chem, 55: 1 (1984).
CrossRef
- Achour M. E., Droussi A., Zoulef S., Gmati F., Fattoum A., Belhadj Mohamed A., Zangar H., Spectro. Lett., 41: 6 (2008).
- S.Tewari, A.Bhattacharjee and P.P. Sahay, Assam University Journal of Science andTechnology, 5: 216-219 (2010).
- G Anandha Bau, G.Bhagavannarayana, P Ramaswamy, Jorn of Cryst Growth, 310: 2820-2826 (2008).
CrossRef
- Prasert Akkaramongkolporn, EtsuoYonemochi and Katsuhide Terada, Chem.Pharm. Bull., 48(2): 231-234 (2000).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal