T. Mohan2, P.Vijayadarshan2, J.Venkata Viswanath1, Amarnath Gupta2, N.V. Srinivasarao2 and A. Venkataraman*1,3.
1Material Chemistry Laboratory, Department of Materials Science, Gulbarga University, Kalaburagi-585106, Karnataka, India.
2R and D center, Premier explosive Limited, P. O. Peddakandukur-508286, Yadadri-Bhongir District, Telangana, India.
3Department of Chemistry, Gulbarga University, Kalaburagi-585106, Karnataka. India.
Corresponding Author Email: raman.dms@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140107
Article Publishing History
Article Received on : 5 Dec 2016
Article Accepted on : 21 Feb 2017
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
This manuscript shows the recrystallization of micro Pentaerythritol tetranitrate (mcPETN) through solvent anti solvent technique by changing some important parameters viz., rotations per minute, temperature of crystallization, while keeping the concentration of PETN constant as in solvent acetone. The synthesized mcPETN is characterized for its crystal structure and morphology. The enhanced performance to its macro sized PETN is reported herewith the results.
KEYWORDS:
Crystal engineering; Impact sensitivity; McPETN; Primary explosive; Solvent anti-solvent crystallization
Copy the following to cite this article:
Mohan T, Vijayadarshan P, Viswanath J. V, Gupta A, Srinivasarao N. V, Venkataraman A. Characterization and Performance Evaluation of Recrystallised Micro PETN. Mat.Sci.Res.India;14(1)
|
Copy the following to cite this URL:
Mohan T, Vijayadarshan P, Viswanath J. V, Gupta A, Srinivasarao N. V, Venkataraman A. Characterization and Performance Evaluation of Recrystallised Micro PETN. Mat.Sci.Res.India;14(1). Available from: http://www.materialsciencejournal.org/?p=5194
|
Introduction
PETN is one of the high energy materials having oxidizer and fuel in the same molecule1. PETN is used as a secondary explosive. Using a different primary explosive to initiate the secondary has its limitations such as, problems in synthesizing high sensitive primary explosives, heterogeneity etc. PETN is considered to have medium sensitivity towards the impact and electric shock.
Coarsening of PETN is the main drawback that limits PETN applicability in the detonators. Doping impurities has managed the problem of aging and coarsening.2 But the impurities may alter the thermal behavior of PETN.3,4 Specific surface area of PETN is the key parameter in choosing PETN in exploding bridge wire (EBW) detonator.5 High surface area PETN production through crash precipitation was reported by Pitchimani et al.4 Changes in the material bulk alter the response towards impulses.
Single crystal of PETN was developed by Meiyu Zhai and Gregory B. McKenna in 2016. The authors have explored the elastic property of PETN single crystal using nano-indentation test.6 A brief study on application of PETN in demolishing works and under water blasts in the form of plastic explosives are dealt by Ahmed Elbeih.7 Semtex is the combination explosive which is made by mixing PETN and RDX in different proportions.8
The crystal engineering techniques comes forth to play a key role in removing the problems that arise due to crystal defects. The crystal size reduction is the technique we adopted in this work. The reduction in crystal size may enhance the surface area and change the morphology to less sensitive.
Solvent anti-solvent crystallization technique is one of the most adopted techniques because the crystals obtained are uniform in size and morphology. Achieving super saturated solution and finally the uniform crystals through the solvent anti solvent method is the basic principle.9
Experimental:
Materials and Methods
The commercial macro PETN is obtained from the Premier Explosives Limited, Hyderabad, India. Acetone and distilled water are taken as solvent and anti solvent respectively in the ratio of 1:10. The commercial macro PETN is mixed with acetone to form a uniform solution. The above solution is added drop by drop to water. The fine crystals of PETN are appeared in the water.
Characterization
Recrystallized final powder is characterized for average particle size (APS) and specific surface area (SSA) using Malvern instrument and Micromeritics instrument respectively. Crystal density of the final powder is calculated manually. Final powder is characterized further for its particle morphology through SEM. Olympus BX-51 instrument is used to get the SEM image.
Functional Tests
The synthesized mcPETN is tested for its performance. Electric firing test is carried out at 5.2kV by placing 1g of mc PETN over an aluminium plate of 3mm thickness.
Results and discussion
This report included the recrystallization of mcPETN at two RPMs viz. 500, 1500 RPM, two temperatures viz. -3.0± 1.00C and 5.0 ±1.00C. The crystal density, APS and SSA of commercial macro PETN and recrystallized mcPETN are tabulated in table 1.
Table 1: Crystal densities, APS and SSA details of Macro PETN and mcPETN at different temperatures and RPMs
|
S.No
|
PETN (g)
|
RPM
|
Temperature(0C)
|
Crystal density (gm/cc)
|
APS (µm)
|
SSA
(m2/g)
|
|
Macro PETN
|
|
|
|
1.76
|
446.4
|
0.052
|
|
01
|
1
|
500
|
-3.0 ± 1.00C
|
1.67
|
21.24
|
1.134
|
|
02
|
1
|
1500
|
-3.0 ± 1.00C
|
1.53
|
12.74
|
1.746
|
|
03
|
1
|
500
|
5.0 ± 1.00C
|
1.42
|
23.52
|
1.084
|
|
04
|
1
|
1500
|
5.0 ± 1.00C
|
1.69
|
13.43
|
1.640
|
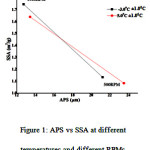
The plot between APS and SSA (figure 1) shows the dependence relation between APS and SSA of mcETN.
Fig 1: APS vs SSA at different temperatures and different RPMs
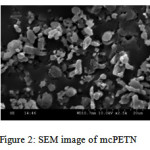
The SEM image of mcPETN is given in figure 2. The agglomerations in the SEM image are possibly due to the presence of water. During the synthesis of mcPETN, the rods like crystals have changed their morphology to spherical, thus improving the SSA. The rods like crystals appeared in figure 2 are less in size when compared with the commercial macro PETN. The spherical crystals have high amount of hotspots in the solid matrix than the regular rod like crystals.10
Fig 2: SEM image of mcPETN
The electric firing test results a hole on the aluminium plate with 3mm thickness as shown in figure 3, while the commercial macro PETN did not initiate the firing. Reduced APS and availability of extra SSA to the electric stimulus may have caused the explosion. The increased hotspots in the solid matrix of the mcPETN crystals are also be responsible for the reactivity towards low electric impulses (5.2kV).
Fig 3: Hole formed on aluminium pate in electric firing test.
Conclusion
The PETN crystallized during solvent antisolvent crystallization technique was observed to be in micro size. The APS, SSA graph has revealed the mc PETN observance. SEM image shows the conversion of rod like crystals to spherical crystals which may lead to the high SSA eventually to its activity towards low electric impulse. The replacement of metallic primary explosives with mcPETN in the manufacturing of armaments where the PETN is being used as a secondary explosive is proposed.
Acknowledgement
The authors are highly thankful to Dr. N.V. Srinivasarao in particular for the technical support. We are also thankful for the M/s. Premier Explosives Limited for providing the R&D facility to carry all the experiments.
References
- Zepeda-Ruiz L. A., Amitesh M., Richard G., George H. G and Brandon L.W. Journal of crystal growth. 2006;291:461-467.
CrossRef
- R.Pitchimani L. J., Hope-Weeks G. Z and Weeks B. L. Journal of energetic materials. 2007;25:203-212.
CrossRef
- Rogers R. N and Dinegar R. H. Thermochimica Acta. 1972;3:367–378.
CrossRef
- Pitchimani R., Zheng W., Simon S., Hope-Weeks L., Burnham A. K and Weeks B. L. Journal of Thermal Analysis and Calorimetry. 2007;89:475–478.
CrossRef
- Young S. Method Development and Validation for Measuring the Particle Size Distribution of Pentaerythritol Tetranitrate (PETN) Powders, Report SAND.2005-6487. Sandia National Laboratories, Albuquerque. 2006.
- Zhai M and Gregory B. M. Crystal research & technology. 2016;51(7):414-427.
CrossRef
- Elbeih A. Journal of Chemistry. 2015:1-6.
CrossRef
- Chambers D. M., Brackett C., Sparkman D. O. Perspecives on Pentaerythritol tetranitrate (PETN) decomposition, UCRL-ID-148956, Lawrence Livermore National Lab., CA (US).
- Genck W. Make the most of anti-solvent crystallizations, http://www.chemicalprocessing.com/articles/2010/210/.2010.
CrossRef
- Venkata J.V., Venugopal K. J.,Srinivasa N. V. R and Venkataraman A. Defence Technology. 2016;12(5):401-418.
CrossRef
CrossRef
Views: 1,277
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal