In-Situ Preparation of Conducting Polymers/Copper(II)-Maghnite Clay Nanocomposites
Fatima Zohra Zeggai1,2*  , Mohammed Belbachir 2 and Aicha. Hachmaoui3
, Mohammed Belbachir 2 and Aicha. Hachmaoui3
1Centre de Recherche Scientifique et Technique en Analyses Physico-chimiques (CRAPC), BP 384-
2Polymer Chemistry Laboratory, Oran1 University Ahmed Benbella, BP 1524, El’Menouer, 31000 Oran, Algeria
3Organic, Macromolecular and Materials Chemistry Laboratory; Mustapha Stambouli University Mascara. BP 763, Street of Mamounia, 29000 Mascara, Algeria
Corresponding author Email: zeggai.fatima-zohra@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140219
Article Publishing History
Article Received on : 30 Aug 2017
Article Accepted on : 15 Sept 2017
Article Published : 22 Nov 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this work we report a simple way for the conducting polymer nanocomposites synthesis using on algerian hydrophilic natural Montmorillonite (MMT) nanoclay named Maghnite (Mag) as dopant. The electrochemical properties study of the following conducting polymers: poly(4-aminobenzylamine) (P4ABA) and polyaniline (PANI) nanocomposites with copper maghnite (Mag-Cu) were successfully prepared by In-Situ polymerization, in presence of inorganic nanolayers of clay, and oxidizing agent ammonium persulfate. The synthesis of copolymers was developed at different feed mole fractions of monomer. The products were characterized by the Fourier transform Infrared (FT-IR), the ultraviolet-visible (UV–vis) spectroscopies and X-ray diffraction (XRD). The results showed that the in-situ polymerization produced real nanocomposites containing aniline and 4-aminobenzylamine units.
KEYWORDS:
Intercalative polymerization; Maghnite clay; Nanocomposites; Polyaniline; Poly(4-aminobenzylamine)
Copy the following to cite this article:
Zeggai F. Z, Belbachir M, Hachmaoui A. Structural, In-Situ Preparation of Conducting Polymers/Copper(II)-Maghnite Clay Nanocomposites. Mat.Sci.Res.India;14(2)
|
Copy the following to cite this URL:
Zeggai F. Z, Belbachir M, Hachmaoui A. Structural, In-Situ Preparation of Conducting Polymers/Copper(II)-Maghnite Clay Nanocomposites. Mat.Sci.Res.India;14(2). Available from: http://www.materialsciencejournal.org/?p=6308
|
Introduction
Recently, clay/polymer nanocomposites have aroused high interest, both in industry and in academia1,2 especially polymer-layered silicate nanocomposites. These nanocomposites are currently prepared by various methods: in-situ polymerization, intercalation from a polymer solution, and direct intercalation by molten polymer (melt compounding).3 The growing interest in polymer nanocomposites initiates from the improvement of enhanced mechanical, flame retardant, gas barrier and polymer recycling properties4 and also, the development of new materials to find potentiel applications.
At present, Montmorillonite is a hydrophilic clay mineral of the smectites group with a 2:1 aluminosilicate layered structure and it can be used in the preparation of polymer nanocomposites (PNCs). The use of MMT in PNCs due to their unique characteristic of intercalation and exfoliation in the polymeric matrixes.5 MMT can be used in the polymer/Clay nanocomposites preparation, only with MMT-Surface modification to make her compatible with hydrophobic polymers. It was generally needed ion exchange reaction between cationic surfactants. However, the MMT-modification allows enlarging the clay interlayer distance. Due to this interlayer space enlargement, polymer chains can enter in the MMT-Clay gallery and can enhanced properties including improved physical and chemical environment for the polymer.6
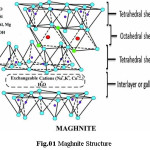
Maghnite (Mag) is Algerian Montmorillonite (MMT) sheet silicate clay with very high cation exchange property.7 She is a natural and non-toxic montmorillonite with a lamellar structure, which was developed at the Polymer Chemistry Laboratory of the University of Oran1 Ahmed Ben Bella,
Algeria.8 Montmorillonites are phyllosilicates minerals comprising a crystal structure of TOT layer (Fig.01), they are specific clays showing both Bronsted and Lewis acid sites9 and they own net negative charge can be neutralized by cations such as Na+, K+, Ca2, etc., which occupy the interlamellar space,10 which can be intercalated or exfoliated, then, industrial applications are all quite dependent on the dispersion of MMT in polymer matrix.
The choice of the polymers is usually guided mainly by their mechanical, thermal, electrical, optical and magnetic behaviors. Hence, electronically conducting polymers have gained a special status owing to wealth of applications. Polyaniline (PANI) and derivative of Polyaniline, have special status among other conducting polymers due to its non-redox doping, good environmental stability and economic feasibility.11-12 They have found wide applications in various research and industry areas.
In this study, we report the cations exchange inside the Maghnite interlamellar space using copper(II) cations. These inorgano-modified Mags were also used to prepare polyaniline/Mag-Cu, Poly(4amino benzylamine)/Mag-Cu and their copolymers/Mag-Cu nanocomposites by In-Situ polymerization. The increased compatibility between the polymers and clay was achieved by production of the nanocomposites containing polymer chains.
Figure 1: IR-FT spectra of the Mag-Na, Mag-Cu and the nanocomposites (P4ABA/Mag-Cu, PANI/Mag-Cu and P(4aba-co-ani)/Mag-Cu).
Experimental
Materials
4-Amino benzylamine (4ABA) and Aniline were purchased from Aldrich Co. Ammonium persulfate ( Merck). A natural montmorillonite clay (named Maghnite) used was supplied by a local company (ENOF Maghnia-ALGERIA).
Modification of Maghnite (Mag-Cu)
The raw-maghnite (Mag-Na) 10 g was crushed for 30 min then dried for 24 h and stored for later use.13 Mag-Cu was prepared by Cu2+ -exchanged reaction. Ten grams of the Mag treated were dispersed in 1M CuSO4 solution, the mixture was stirred for 24h, this product was then filtered and
given several washings with distilled water, to eliminate SO4−. The product was exsiccated at 110 ◦C, overnight and its composition was determined by X-ray fluorescence (Table1).
Polymer/Mag-Cu Nanocomposites synthesis
Polymer/Mag-Cu Clay nanocomposites were synthesized by firstly the intercalation of Copper-Maghnite (0.25 g), then utilizing 4-amino benzyl amine and/or aniline monomers.
The monomers were appended by different mole fractions. In all preparations, the mole ratio of oxidant to the total monomer was 0.5. For instance, the method for poly(aniline-co-4-amino benzylamine)/Mag-Cu nanocomposites mole fraction for f1=0.50 were as fellow; aniline (0.39g, 4.2mmol), 4-amino benzyl amine (0.25g, 4.3mmol) were added to Mag-Cu, and the mixture was kept under stirring and heated at 60°C. A solution of ammonium persulfate (0.49g, 4.1mmol) dissolved in 100ml distilled water, was then added to the reaction mixture. It was stirred for 24h.
Measurements
The XRD patterns of the obtaining nanocomposites were carried out at room temperature on a Bruker AXS D8 Advance diffractometer equipped with LynxEye linear detector, with a X-ray generator Cu Kα1 (λ= 1.54056 Å) and operated at (40kV, 40mA). In the range of 2θ = 0°– 70° and scanning speed of 0.02°/s.
X-ray fluorescence spectroscopy of the chemical compositions of Maghnites (Na and Cu) were determined by XRF using a PW 1480 Philips Analytical wavelength-dispersive sequential XRF spectrometer with Super Q P analytical software.
The Ultraviolet-Visible (UV-Vis) optical absorption spectra were performed using a Hitachi U-3000 spectrophotometer with N-methyl-2-pyrrolidone (NMP) solution. The Fourrier transform infrared were recorded on a Bruker alpha spectrometer (over the range 400 – 4000 cm-1).
Voltammetric measurements are carried out using an electrochemical cell made up of three electrodes immersed in a solution containing the analyte and an excess of a non- reactive electrolyte called the supporting electrolyte. One of the three electrodes is the working electrode, which is in this work made of glassy carbon. The redox process occurs at this electrode. The second electrode is the reference electrode, which provides calibration for the applied potential. Example of this work the reference used was the reversible hydrogen electrode (RHE). The third electrode is the counter electrode, which is often a platinum wire that simply serves to conduct electricity from the signal source through the solution to the other electrodes.
The cell electrolyte was 1M HClO4, and all measurements were carried out at 0.50 V/s, after extraction the polymers using NMP (N-Methyl-2-pyrrolidone).
Characterization Results and Discussion
Maghnite structure composition
Table 1 shows the chemical compositions of the Maghnites determined by X-ray fluorescence spectrometry (XRF). It is necessary to note that Maghnite-Na shows the increase of the content of Na2O, and cation exchange of copper in the Mag-Cu is detected by the appearance of the content of CuO.
Table 1: Chemical analysis of Maghnites.
|
|
Chemical Compositions wt.%
|
SiO2
|
Al2O3
|
Fe2O3 CuO
|
Na2O
|
MgO TiO2 P2O5
|
ZrO2
|
|
|
Mag-Na
|
72.77
|
24.15
|
1.95
|
–
|
2.66
|
3.37
|
0.17
|
0.01
|
0.01
|
|
|
Mag-Cu
|
67.66
|
17.17
|
1.78
|
2.15
|
0.01
|
2.56
|
0.11
|
–
|
0.01
|
Nanocomposites Characterization
FT-IR spectroscopy
The representative FTIR spectra of the maghnite before and after ion exchange and attachment of polymers chains in the nanocomposites were given in Fig.1 and Table 2. The peaks between 3500 and 3100 cm-1 (3351 and 3219 cm-1 for the P4ABA/Mag-Cu, 3337 cm-1 for the PANI/Mag-Cu, and 3340 cm-1 for the P(4aba-co-ani)/Mag-Cu (50/50) nanocomposites associated to N-H bending. Broad absorption peaks between 1580 and 1400 cm-1 from the spectrum of P(4aba-co-ani)/Mag-Cu and P4ABA/Mag-Cu nanocomposites are typical for secondary amines. Absorption above 1600 cm-1 from the spectrum of P4ABA/Mag-Cu nanocomposites correspond the presence of C=N.14
The Peaks between 1040 and 450 cm-1 indicate the Mag clay inside nanocomposites, while the peaks at 1487 cm-1, 1448 cm-1 and 1310 cm-1 for PANI/Mag-Cu and P(4aba-co-ani)/Mag-Cu (50/50) nanocomposites correspond to correspond to quinone- and benzene-ring stretching deformations, respectively.15
Table 2: Important IR bands of Montmorillonites (Mag-Na, Mag-Cu) and Nanocomposites: (a) PANI/Mag-Cu; (b) P(4aba-co-ani)/Mag-Cu (50/50); (c) P4ABA/Mag-Cu.
|
Samples
|
Wave number range (cm-1)
|
Assignments
|
Refs
|
|
Mag-Na, Mag-Cu
|
3620
|
|
|
O-H str. (hydroxyl group)
|
[16-20,22]
|
|
|
3385, 3418
|
|
H-OH Hydrogen bonded water
|
[22]
|
|
|
1630
|
|
|
O-H deformation of entrapped water
|
[22]
|
|
|
996, 1000
|
|
Si-O in-plane str.
|
[22, 24]
|
|
|
515
|
|
|
(Si-O-Al) deformation
|
[22,24]
|
|
Nanocomposites
|
3337-3351 (a, b, c)
|
N-H str.
|
[22-25]
|
|
|
1580-1400
|
(b, c)
|
Vibration of secondary amine
|
[14]
|
|
|
1600
|
(c)
|
|
C=N vibration
|
[14]
|
|
|
1043
|
(c)
|
|
1,2,3-trisubstitution of 4-aminobenzylamine
|
[27]
|
|
|
1573, 1586
|
(a, b)
|
C=C str. vibrations of quinoid ring
|
[15,26]
|
|
|
1487, 1498 (a, b)
|
C=C str. vibrations of benzoid ring
|
[15,26]
|
|
|
1266
|
(a, b)
|
|
C-N str. of secondary aromatic amine
|
[15,25-26]
|
|
|
1011-1040 (a, b, c)
|
Si-O in-plane str.
|
[15,21]
|
|
|
515 (a, b, c)
|
|
(Si-O-Al) deformation
|
[22,24]
|
Ultraviolet-Visible absorption measurements
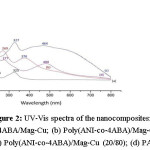
Fig 2. Show typical Ultraviolet-Visible spectra of homo- and co-polymer nanocomposites doped with Mag-Cu. However, the absorption depends on the polymerization conditions and the medium in which the film is examined. For the homopolymers nanocomposites (spectra (a) and (d) for P4ABA/Mag-Cu and PANI/Mag-Cu, respectively) two characteristic peaks, can be observed at about
234/325 nm and 277/480 nm, corresponds to the → π* transition and to the π→ π* transition, respectively, these transition-related absorbance peaks clearly revealed the transition of the benzenoid rings and quinine-imine groups.
For the copolymers/Mag-Cu nanocomposites two major absorption peaks, can be seen in the spectra and (c) at about 269/376 nm and 327/464 nm. These peaks are due to the presence of the –CH2-NH2 groups in the copolymer chain. The absorption results obtained from electrochemical polymerization of the mixture of Aniline and 4-Aminobenzylamine clearly show the incorporation of monomers into the polymer during polymerization.
Figure 2: UV-Vis spectra of the nanocomposites: (a) P4ABA/Mag-Cu; (b) Poly(ANI-co-4ABA)/Mag-Cu (80/20); (c) Poly(ANI-co-4ABA)/Mag-Cu (20/80); (d) PANI/Mg-Cu.
XRD spectroscopy
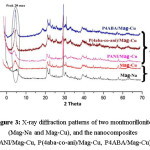
Figure 3: X-ray diffraction patterns of two montmorillonite (Mag-Na and Mag-Cu), and the nanocomposites (PANI/Mag-Cu, P(4aba-co-ani)/Mag-Cu, P4ABA/Mag-Cu).
The X-ray diffraction of Mag-Na, Mag-Cu and nanocomposites (PANI/Mag-Cu, P(4aba-co-ani)/Mag-Cu, P4ABA/Mag-Cu) are shown in Fig.3 and quantitative data are given in Table 3. The change in the interlayer spacing of maghnite after ion exchanging Na+ with Cu++ was found to increase from 12.79Å in the Mag-Na to 14.60Å in the Mag-Cu. In the case of nanocomposites, the repetitive multilayer structure is well preserved, allowing the interlayer spacing to be determined. The intercalation of the polymer chains usually increases the interlayer spacing, in comparison with the spacing of the modified Clay used. The interlayer spacing was estimated according to Bragg’s diffraction formula: λ= 2d sinϴ, where λ is the X-ray wavelength and 2ϴ is the scattering angle. X-ray diffraction (XRD) patterns were obtained with a Japanese Rigaku D/max X-ray diffractometer equipped with graphitemonochromatized Cu K radiation (λ = 1.54 Å).
Table 3: Basal spacing (d(001)) of Maghnite-Na, Maghnite-Cu and Nanocomposites
|
Samples
|
2ϴ max (deg)
|
d(001) (A°)
|
∆d (A°)
|
|
|
|
|
|
|
Mag-Na
|
6.95
|
12.79
|
–
|
|
Mag-Cu
|
6.09
|
14.60
|
1.81
|
|
P(4aba_co-ani)/Mag-Cu (50/50)
|
5.84
|
15.32
|
2.53
|
|
P4ABA/Mag-Cu
|
5.72
|
15.63
|
2.84
|
|
PANI/Mag-Cu
|
5.98
|
14.88
|
2.09
|
|
|
|
|
|
Electrochemical response
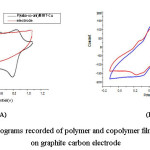
Figure 4: Cyclic voltammograms recorded of polymer and copolymer films formed in 1.0 M HClO4 on graphite carbon electrode
Comparative cyclic voltammetric (CV) of polymer and copolymer films obtained from P(4aba-co-ani)/Mag-Cu (20/80) at a graphite carbon electrode in 1 M HClO4 solution at a scan rate of 50 mV s-1, is shown in Figures 5A and 5B. The significance of this study was to explore the best electron transport properties of obtaining nanocomposites. In the case of PANI/MMT-Cu, two overlapped redox processes are observed. The first one appears at 0.32/0.26 V, which results in which results in a potential peak separation (∆Ep) close to 60 mV; the second process is observed at 0.63/0.48 V and gives an ∆Ep value of 150 mV. This redox process corresponding to leucoemeraldine/emeraldine and emeraldine/pernigraniline transitions, respectively [28]. On the contrary, the P4ABA/MMT-Cu shows the electrochemical response that is only one redox peak at 0.32/0.49 V. This can be due to the differences in the structures of the polymer.
On the other hand, the nanocomposite P(4aba-co-ani)/Mag-Cu (20/80) displayed different redox properties that hinged on the other samples synthesized. Which present two good and higher redox processes at 0.34/0.47 V and 0.79/0.84 V. This redox process leads to the dependence on the amounts PANI of nanocomposite.
Therefore, the comparative cyclic voltammetry studies reveal that the best electron transport properties is the nanocomposite P(4aba-co-ani)/Mag-Cu (20/80) due to the presence of polar functional groups that may promote covalent cross-link chains in copolymers.
Conclusion
In this study, a poly(4-aminobenzyl amine) (P4ABA), polyaniline (PANI), and copolymer (4aba-co-ani) doped with copper-maghnite clay nanocomposites was successfully synthesized by In-Situ polymerization in the presence of ammonium persulfate a oxidant. The X-ray diffraction, FT-IR spectra and UV-Vis spectroscopy shows some peaks shifts, which indicate the formation of some new bonds and support the intercalation of polymer chains into the interlayer spacing of maghnite (Mag-Cu). Good electrochemical response has been observed for copolymers grown into Mag-Cu in which the cyclic voltammogram shows a broad anodic peak that consists on the overlapping of one redox processes; this indicates that the copolymerisation into Mag-Cu is electroactive.
References
- Alexandre M & Dubois P. Polymer-layered silicate nanocomposites: preparation. 2000.
- properties and uses of a new class of materials. Materials Science and Engineering: R: Reports. 28(1):1-63.
- Beyer G. (Ed.) Industry Guide to Nanocomposites, Applied Market Information Ltd, Bristol, UK. 2009.
- Komarneni S. Nanocomposites. Journal of Materials Chemistry. 1992;2(12):1219-1230.
CrossRef
- Yi D., Yang H., Zhao M., Huang L., Camino G., Frache A & Yang R. A novel, low surface charge density, anionically modified montmorillonite for polymer nanocomposites. RSC Advances. 2017;7(10):5980-5988.
CrossRef
- Zhang G., Wu T., Lin W., Tan Y., Chen R., Huang Z & Qu J. Preparation of polymer clay nanocomposites via melt intercalation under continuous elongation flow. Composites Science and Technology. 2017;145:157-164.
CrossRef
- Stejskal J & Gilbert R. G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure and Applied Chemistry. 2002;74(5):857-867.
CrossRef
- Belbachir M., Bensaoula A. Composition and method for catalysis using bentonites. US. 2006;7094823B2.
- Bennabi S & Belbachir M. Synthesis and Characterization of New Organometallic Hybrid Material LCP-1 Based on MOF (Metal–Organic Framework) and Maghnite-H+, a Protons Exchanged Montmorillonite Clay, as Catalytic Support. Journal of Inorganic and Organometallic Polymers and Materials. 2017;1-13.
CrossRef
- Bennabi S., Sahli N., Belbachir M., Brachais C. H., Boni G & Couvercelle J. P. New approach for synthesis of poly (ethylglyoxylate) using Maghnite-H+, an Algerian proton exchanged montmorillonite clay as an eco-catalyst. Journal of Macromolecular Science, Part A. 2017;1-10.
- Zhou Y., Li F., Liu J., Yun Z & Gui X. Pt-H 2 SO 4/Zr-montmorillonite: An efficient catalyst for the polymerization of octamethylcy-clotetrasiloxane, polymethylhydrosiloxane and hexamethyldisiloxane to low-hydro silicone oil. Chinese Journal of Chemical Engineering. 2017.
CrossRef
- Borsenberger P. M & Gruenbaum W. T. Electron transport in a molecularly doped polymer. Journal of Polymer Science Part B: Polymer Physics. 1996;34(3):575-582.
CrossRef
- Amano K., Ishikawa H., Kobayashi A., Satoh M & Hasegawa E. Thermal stability of chemically synthesized polyaniline. Synthetic metals. 1994;62(3):229-232.
CrossRef
- Toumi I., Benyoucef A., Yahiaoui A., Quijada C & Morallon E. Effect of the intercalated cation-exchanged on the properties of nanocomposites prepared by 2-aminobenzene sulfonic acid with aniline and montmorillonite. Journal of Alloys and Compounds. 2013;551:212-218.
CrossRef
- Thiemann C & Brett C. M. Electrosyn thesis and properties of conducting polymers derived from aminobenzoic acids and from aminobenzoic acids and aniline. Synthetic Metals. 2001;123(1):1-9.
CrossRef
- Lu J & Zhao X. Electrorheological properties of suspensions based on polyaniline-montmorillonite clay nanocomposite. Journal of materials research. 2002;17(06):1513-1519.
CrossRef
- Pramoda K. P., Liu T., Liu Z., He C & Sue H. J. Thermal degradation behavior of polyamide 6/clay nanocomposites. Polymer degradation and Stability. 2003;81(1):47-56.
CrossRef
- Liang Z. M., Yin J & Xu H. J. Polyimide/montmorillonite nanocomposites based on thermally stable, rigid-rod aromatic amine modifiers. Polymer. 2003;44(5):1391-1399.
CrossRef
- Madejová J. FTIR techniques in clay mineral studies. Vibrational spectroscopy. 2003;31(1):1-10.
CrossRef
- Magaraphan R., Lilayuthalert W., Sirivat A & Schwank J. W. Preparation, structure. 2001.
- properties and thermal behavior of rigid-rod polyimide/montmorillonite nano composites. Composites Science and Technology. 61(9):1253-1264
- Liu W., Ni, Y., & Xiao H. Montmorillonite intercalated with polyaminoamide– epichlorohydrin: preparation, characterization and sorption behavior. Journal of colloid and interface science. 2004;275(2):584-589.
CrossRef
- Belmokhtar A., Benyoucef A., Zehhaf A., Yahiaoui A., Quijada C & Morallon E. Studies on the conducting nanocomposite prepared by polymerization of 2-aminobenzoic acid with aniline from aqueous solutions in montmorillonite. Synthetic Metals. 2012;162(21):1864-1870.
CrossRef
- Katti K. S., Sikdar D., Katti D. R., Ghosh P & Verma D. Molecular interactions in intercalated organically modified clay and clay–polycaprolactam nanocomposites: experiments and modeling. Polymer. 2006;47(1): 403-414.
CrossRef
- Wan C., Zhang Y & Zhang Y. Effect of alkyl quaternary ammonium on processing discoloration of melt-intercalated PVC-montmorillonite composites. Polymer Testing. 2004;23(3):299-306.
CrossRef
- Sudha J. D., Sivakala S., Chandrakanth C. K., Neethu K. S., Rohini K. N & Ramakrishnan. Percolated conductive polyaniline-clay nanocomposite in polyvinyl chloride through the combined approach porous template and self-assembly. Express Polymer Letters. 2014;8(2):107-115.
CrossRef
- Ragachev A. A., Yarmolenko M. A., Xiaohong J., Shen R., Luchnikov P. A & Rogachev A. Molecular structure, optical, electrical and sensing properties of PANI-based coatings with silver nanoparticles deposited from the active gas phase. Applied Surface Science. 2015;351:811-818.
CrossRef
- Kong L., Lu X., Jin E., Jiang S., Bian X., Zhang W & Wang C. Constructing magnetic polyaniline/metal hybrid nanostructures using polyaniline/Fe 3 O 4 composite hollow spheres as supports. Journal of Solid State Chemistry. 2009;182(8):2081-2087.
CrossRef
- Rivas B. L & Sanchez C. O. Poly (2‐) and (3‐aminobenzoic acids) and their copolymers with aniline: Synthesis, characterization, and properties. Journal of applied polymer science. 2003;89(10):2641-2648.
CrossRef
- dos Poli A. K. S., Caetano G. M. D., Vargas L. R., Gama A. M., Baldan M. R & Gonçalves E. S. Electrosynthesis of Polyaniline on Carbon Fiber Felt: Influence of Voltammetric Cycles on Electroactivity. Journal of The Electrochemical Society. 2017;164(9):D631-D639.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Mohammed Belbachir 2 and Aicha. Hachmaoui3
, Mohammed Belbachir 2 and Aicha. Hachmaoui3 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal