Introduction
Free radicals and reactive oxygen species (ROS) are very reactive molecules that are produced by cells during respiration and cell-mediated immunological responses. The disease will develop if cellular defenses against reactive oxygen species are ineffective. Antioxidants are essential to prevent oxidative damage because different disease processes can occasionally thwart these defense mechanisms 1. Medicinal plants have been an integral part of human life since the dawn of civilization 2,3. The discovery of traditional medicines with significant antioxidant potential has received much attention in recent years 4,5.
Azadirachta indica (neem) is well known in India and its neighboring countries as one of the most versatile medicinal plants with a broad spectrum of biological activity and India’s most commonly used traditional medicinal plant for household remedies against various human ailments. A. indica contains various primary compounds, including fat derivatives, carbohydrates, and proteins, and secondary compounds, such as flavonoids, steroids, saponins, terpenoids, alkaloids, glycosides, and tannins 6. Phyllanthus emblica (Amla) has been used in Indian traditional medicine for the treatment of several diseases, such as hemorrhage, diarrhea, jaundice, dyspepsia and pharmacological investigations support the antioxidant activities of different extracts 7. Psidium guajava (guava) is used in traditional medicine against diarrhea, diabetes, and stomach aches. It possesses pharmacological activities like antispasmodic, cough sedative, anti-inflammatory, antidiarrheic, antihypertension, antiobesity, and antidiabetic properties 8. Mentha piperita (Peppermint) is known for antioxidant, cytotoxic, and antibacterial activities with fewer side effects. It has also been used as a headache and migraine medicine and a treatment for intestinal colic, liver diseases, gastritis, and jaundice 9. Ocimum tenuiflorum (Tulsi) has many biological properties, including analgesic, antiemetic, hypoglycemic, immune-booster, antibacterial, stress-relieving, and expectorant effects. The antioxidant potential of O. tenuiflorum is due to various secondary metabolites, including phenolics and flavonoids 10. Syzygium cumini (Java Plum) is a purple-red to dark fruit containing large amounts of phytonutrients like flavonoids known for their anti-inflammatory, antidiabetic, anticancer, radical scavenging, and antioxidant potential 11.
A diverse range of medicinal properties like antioxidant, anti-inflammatory, antitumorigenic, anticarcinogenic, and antidiabetic activities of herbs and spices is due to the presence of tannins, alkaloids, phenolics, flavonoids, and polyphenols 12. Allium sativum (garlic) sulfur-containing compounds are responsible for anti-inflammatory, anticancer, antitumor, antidiabetic, and cardioprotective properties. The majority of sulfur-containing compounds are garlic thiosulfate (allicin), S-allyl cysteine sulfoxide (alliin), ajoenes (E- and Z-ajoene), vinyldithiins (2-vinyl-(4H) -1,3-dithiin, 3-vinyl-(4H)-1,2-dithiin), and diallyl (di and tri) sulfide 13. Allium cepa (Onions) are considered an excellent antioxidant source and contain a significant amount of flavonoids and phenols. Polyphenols are phenol derivatives with a high antioxidant capacity because they effectively eliminate reactive oxygen species and have important anti-inflammatory actions. Quercetin and its glycosylated derivatives in A. cepa are anticipated to have an antioxidant potential 14-17. Dalbergia sissoo is rich in dalberginone, dalbergin, isoflavone-O-glycoside, 7, 4dimethyle tectorigenin, isocaviumin, etc., and known for antioxidant and antidiabetic activities. Syzygium aromaticum (Cloves) is used primarily in food, perfume, cosmetics, and medicinal products. S. aromaticum has different pharmacological and antibacterial properties because of its constituents, such as glycosides, hormones, tannins, alkaloids, and saponins 18.
The antioxidant potentials of many medicinal plants have been exploited in different ways. The ability to scavenge free radicals and reactive oxygen species has been evaluated using various techniques based on spectrophotometry and chemiluminescence19. The relationship between plant phenolic content and antioxidant activity was measured by the 2,2-diphenyl-1-picrylhydroazyl (DPPH) and Nitric Oxide (NO) Radical Scavenging assays 20. The medicinal plants chosen for this study are Phyllanthus embelica, Mentha piperita, Ocimum tenuiflorum, Azadirachta indica, Syzgium aromaticum, Dalbergia sissoo, Allium sativum, Psidium guajava, Syzygium cumini, and Allium cepa. The ten selected plants are commonly used in the Indian traditional medicine system due to their potential health-promoting and pharmacological qualities. Previous studies have reported the antioxidant potential of selected plant extracts, but the comparative evaluation is lacking. The current study aimed phytochemical screening of ten selected medicinal plants and to analyze the antioxidant activities under the same evaluation condition. The screened extracts of medicinal plants can be used as potential antioxidant agents and resources for developing polyherbal formulations against various inflammation-related diseases.
Methodology
Sample collection and preparation
Fresh plant leaves from Phyllanthus embelica (Pe), Mentha piperita (Mp), Ocimum tenuiflorum (Ot), Azadirachta indica (Ai), Dalbergia sissoo (Ds), Allium sativum (As), Psidium guajava (Pg), Syzygium cumini (Sc) and Allium cepa (Ac) were harvested from the Shobhit Institute of Engineering & Technology, (Deemed-to-be-University), Modipuram, Meerut, India. Syzgium aromaticum (Sa) flower buds were obtained from the local market (Vedaka Spices and Seeds, Karnataka, India, FSSAI LIC No. 10018011005884). The samples were rinsed with water and then shade dried until all moisture content was gone. The plant samples were adequately cleaned before being ground into an incredibly fine powder using a household blender. The final dried sample was stored under a vacuum for further experiments.
The hydroalcoholic extract was prepared by mixing 20 ml of 70 % ethanol and 2 g of powdered plant samples. Following proper mixing, plant samples were incubated for one week at room temperature for extraction. The samples were thoroughly stirred and centrifuged at 4000 rpm for 15 minutes. The supernatant was filtered with Whatman No.1 filter paper, transferred into pre-weighed glass containers, and then dried under laminar flow for further analysis.
UV-VIS Spectrum Analysis
The phytochemicals screening of different plant extracts (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) was conducted using UV-VIS spectrum analysis. 1 g of extract was centrifuged at 3000 rpm for 10 mins and filtered through filter paper. The samples were diluted to 1:10 with the same solvent (70 % ethanol). An aliquot of the diluted sample was scanned using UV-Visible Spectrophotometer at a range of 200-800 nm wavelength (Shimadzu UV-1800, Kyoto, Japan) and the characteristic peaks of each extract were recorded.
Total Antioxidant Capacity
The total antioxidant capacity of selected plant extracts (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) was evaluated by the phosphomolybdate method. The test samples (0.3 ml) was mixed with 3 ml of the reagent solution (0.6 M Sulfuric acid, 28 mM Sodium phosphate and 4 mM Ammonium molybdate). The reaction mixture tubes were incubated at 95 ºC for 90 min. The absorbance of the solution was measured at 695 nm using a UV-VIS spectrophotometer (Shimadzu UV-1800, Kyoto, Japan) against the blank. The total antioxidant activity was expressed as mg Ascorbic acid gram equivalents 21.
DPPH Radical-Scavenging Activity
An antioxidant capacity to scavenge free radicals is routinely evaluated using the DPPH test. In the current study, different concentrations of plant extracts (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) were analyzed. About 10 mL of (0.1 mM) DPPH was prepared in ethanol and 1 ml of DPPH solution dissolved in different extracts of ethanol at various concentrations (0, 25, 50, 75, 100, 500, 750, 1000 µg/ml) was mixed, and the reaction mixture was incubated in the dark for 30 mins at room temperature and the absorbance was recorded at 517 nm. The reaction mixture without extract served as control and L-ascorbic acid was used as an antioxidant standard. The IC 50 value of the sample was calculated based on the absorbance.
The percentage DPPH, the radical scavenging activity of each extract, was done using the formula:
% DPPH radical scavenging activity = ( T0 − T )/ T0 × 100)
where T0 is the absorbance of the control and T is the absorbance of the test sample.
Nitric Oxide Radical Scavenging Assay
The nitric oxide scavenging activity of the selected plant extracts (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) was measured at different concentrations (50, 100, 200, 400, 800, 1000 µg/mL). 100 μl (10 mM) Sodium nitroprusside was prepared in saline phosphate buffer and was added to 100 μl of each extract. Then 1 mL of Griess reagent (prepared by mixing equal volumes of 1% sulphanilamide in 2% phosphoric acid and 0.1% naphthyl ethylenediamine dihydrochloride in water) was added to reaction mixtures, incubated for 3 h.
The absorbance of solutions was measured at 540 nm against the corresponding blank solutions using the following formula:
Nitric oxide radical scavenging = A (blank) – A (sample) × 100 / A (blank)
Where, A (blank) = absorbance of control sample and A sample= absorbance in the presence of the samples or standards.
Statistical analysis
All experiments were performed in triplicate (n=3) and results were expressed as mean±SEM. A
one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison t-test was used to calculate statistical difference. Statistical significance was considered at *p< 0.05.
Results and Discussion
Characterization of hydroalcoholic Plant Extract using UV-VIS Spectrophotometer
UV-VIS spectroscopic is a simple, cost-effective, and rapid test for screening phytochemicals in plant-based extracts 22. UV-VIS spectrophotometry was used to detect the presence of phytochemicals by identifying compounds containing π-bonds, lone pairs of electrons, σ-bonds, aromatic rings, and chromophores in the UV-VIS region on the electromagnetic spectrum ranging from 200 to 700 nm indicating the presence of secondary metabolites such as alkaloids, flavonoids, phenolic compounds, tannins, terpenoids, carotenoids, and chlorophyll 23. Secondary metabolites exhibit a broad spectrum of pharmacological properties, including wound healing, analgesic, anti-inflammatory, antioxidant, and anti-microbial, which numerous researchers have described. The hydroalcoholic plant extracts of ten different medicinal plants (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) were scanned using UV-VIS spectrophotometer in the wavelength ranges from 200 – 800 nm and the characteristic peaks were recorded (Fig. 1). Distinct peaks at 328 and 380 nm were observed in P. embelica spectrum in the absorption range of 1-2 nm. The M. piperita spectra profile showed the peaks at 330, 388, 576, and 638 nm in the absorption range of 3-4 nm. Three distinct peaks at 334, 606, and 658 nm were observed in O. tenuiflorum spectra profile in the absorption range of 3.0-4.0 nm. The peaks at 312, 334, 388, 506, 538, and 656 nm were visible in the A. indica profile in the absorption range of 3-4 nm, and a distinct peak at 608 nm was observed in the absorption range of 2.5-3 nm. The S. aromaticum spectra profile showed the peaks at 328 and 380 nm in the absorption range of 3-4 nm. The peaks at 328 and 380 nm were noted in the D. sissoo spectrum in the absorption range of 1.5-2 nm. The number of peaks obtained from A. sativum spectrum was recorded at 380 and 394 nm in the absorption range of 2.5-3 nm. Distinct peaks at 328 and 664 nm were observed in P. guajava spectra profile in the absorption range of 1.5-2.0 nm and 0-0.5 nm, respectively. S. cumini spectra profile showed the peaks at 328 and 664 nm in the absorption range of 1-2 nm. Similarly, two peaks were recorded in the same absorption range at 328 and 338 nm in the spectra of A. cepa.
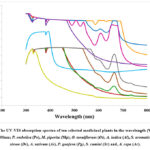 |
Figure 1: The UV-VIS absorption spectra of ten selected medicinal plants in the wavelength (W/L) ranges from 200-800nm;
Click here to View Figure
|
Total Antioxidant Capacity
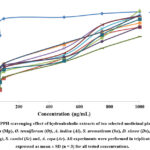
The total antioxidant capacity estimates both water-soluble and fat-soluble antioxidants and involves reduction of phosphomolybdate by the extracts following formation of phosphomolybdenum complex 21. In the present study, the antioxidant capacity of hydroalcoholic extracts isolated from ten selected medicinal plants (P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa) was measured spectrophotometrically at 695 nm and expressed as microgram equivalents of Ascorbic Acid per gram of extract (µgAAE/g). In the present study, the highest antioxidant capacity was observed in M. piperita (202.56 ± 1.98 µgAAE/g) followed by S. aromaticum (201.16 ± 1.78 µgAAE/g), O. tenuiflorum (199.42 ± 0.51 µgAAE/g), A. indica (197.16 ± 1.28 µgAAE/g), D. sisso (175.23 ± 1.53 µgAAE/g), P. embelica (168.62 +0.95 µgAAE/g), S. cumini (165.33 ± 1.91 µgAAE/g), A. sativum (162.01 ± 2.01 µgAAE/g), A. cepa (158.56 ± 0.88 µgAAE/g) and, P. guajava (135.36 ± 1.77 µgAAE/g).
Antioxidant Analysis using DPPH radical scavenging assay
DPPH free radical method has broadly been employed to evaluate the free radical scavenging activity of the natural antioxidants24. In the present study, the antioxidant potential of hydroalcoholic extracts of P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa was evaluated at varied concentrations ranges from 0 – 1000 µg/mL using the DPPH free radical scavenging assay. The percent of DPPH radical scavenging activity at highest tested concentration (1000 µg/mL) was found 77 % in P. embelica with IC50 value of 570 ± 1.13 μg /mL, 94 % in M. piperita with IC50 value of 561 ± 1.13 μg /mL, 89 % in O. tenuiflorum with IC50 value of 420 ± 0.69 μg /mL, 89 % in A. indica with IC50 value of 502 ± 1.11 μg /mL, 81 % in S. aromaticum with IC50 value of 480 ± 1.61 μg /mL, 89 % in D. sissoo with IC50 value of 410 ± 0.62 μg /mL, 88 % in A. sativum with IC50 value of 500 ± 1.60 μg /mL, 67 % in P. guajava with IC50 value of 680 ± 1.26 μg /mL, 77 % in S. cumini with IC50 value of 510 ± 1.23 μg /mL, 85 % in A. cepa with IC50 value of 540 ± 1.16 μg /mL. The free radical scavenging activity of different extracts was in the following order based on maximum tested concentration; M. piperita > A. indica=O. tenuiflorum=D. sissoo > A. sativum > A. cepa > S. aromaticum>S. cumini >P. embelica>P. guajava. The highest IC50 value was demonstarted by O. tenuiflorum and lowest by P. guajava. The antioxidant capacity of the extract was compared with ascorbic acid (IC50 value: 62.12± 0.63 μg /mL) as the standard antioxidant (Fig. 2). The free radical scavenging potential observed in these medicinal plant samples was because of the presence of some natural source such as phenol, flavonoid, or tannin contents. The highest antioxidant capacity in M. piperita might be attributed to the high phenolic content of the plant 25,26.
Nitric Oxide Radical Scavenging Assay
The interaction of nitric oxide (NO) with oxygen and other free radicals, such as superoxide, results in nitric oxide, which is categorized as a free radical based on unpaired electrons. Nitric oxide radicals can cause tissue damage when produced excessively or exposed to them over an extended period 27. In order to treat chronic inflammatory illnesses, researchers have focused more on identifying natural antioxidants that may act as potent NO production inhibitors. Nitric radical scavenging assay was carried out using hydroalcoholic extracts of ten medicinal plants; P. embelica, M. piperita, O. tenuiflorum, A. indica, S. aromaticum, D. sissoo, A. sativum, P. guajava, S. cumini and A. cepa at different concentrations (50, 100, 200, 400, 800, 1000 μg/mL). The increase in antioxidant activity was observed with increase in concentration of the extracts and the maximum free radical scavenging activity at highest tested concentration was found in O. tenuiflorum (89 % inhibition) followed by S. aromaticum (81 % inhibition), M. piperita (77 % inhibition), A. indica (75 % inhibition), D. sissoo (71 % inhibition), S. cumini (68 % inhibition), P. guajava (67 % inhibition), A. sativum (61 % inhibition), A. cepa (59 % inhibition), and P. embelica (59 % inhibition). The Percentage of free radical scavenging activity was plotted against the concentration of the extracts as shown in Fig. 3.
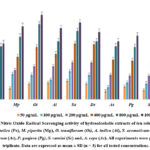 |
Figure 3: The Nitric Oxide Radical Scavenging activity of hydroalcoholic extracts of ten selected medicinal plants;
Click here to View Figure
|
Conclusion
Medicinal plants are a promising source of potent antioxidants and anti-inflammatory agents that may effectively cure several human ailments. Antioxidants help to neutralize free radicals, the primary source of inflammatory disorders. The current study determined the antioxidant potential of different medicinal plants by using different assays, such as DPPH Radical-Scavenging and Nitric Oxide Radical Scavenging Assay. The findings from the present investigation indicated the antioxidant potential of hydroalcoholic extracts of ten medicinal plants. Many inflammatory diseases and disorders can be prevented or treated with the help of medicinal herbs. However, further study is required to identify, characterize and evaluate the bioactive components responsible for therapeutic benefits. Moreover, herbal combinations can also be assessed for potential use in pharmaceutical product development and future therapeutic breakthroughs.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding sources.
References
- Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019; 24:4132.
CrossRef - Saeed M, Naveed M, BiBi J, Kamboh AA, Arain MA, Shah QA, Alagawany M, El-Hack MEA, Abdel-Latif MA, Yatoo MI, Tiwari R, Chakraborty S, Dhama K. The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica Granatum L. (Pomegranate) as a Functional Food in Humans and Animals. Recent Pat Inflamm Allergy Drug Discov 2018;12(1):24-38. doi: 10.2174/1872213X12666180221154713. PMID: 29473532.
CrossRef - Sadhasivam S, Garkhal K, Singh H, Yadav V, Chawrai S, Ramnane M, Jain S, Sardana K, Ghosh S. Newly Developed Anti-Dandruff Regimen, VB-3222, Delivers Enhanced Sensorial and Effective Therapeutic Benefits Against Moderate Adherent Dandruff. Clin Cosmet Investig Dermatol 2020; 21;13:187-195. doi: 10.2147/CCID.S219109. PMID: 32110083; PMCID: PMC7041436.
CrossRef - Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 2008; 8:63. doi: 10.1186/1472-6882-8-63. PMID: 19068130; PMCID: PMC2636748.
CrossRef - Tashim NA, Lim SA, Basri AM. Synergistic antioxidant activity of selected medicinal plants in Brunei Darussalam and its application in developing fortified pasta. J Sci Food Agric 2022 ;102(15):7331-7342. doi: 10.1002/jsfa.12099. Epub 2022 Jul 19. PMID: 35767363.
CrossRef - Pathak RK, Kim DY, Lim B, Kim JM. Investigating Multi-Target Antiviral Compounds by Screening of Phytochemicals From Neem (Azadirachta indica) Against PRRSV: A Vetinformatics Approach. Front Vet Sci 2022; 9:854528. doi:10.3389/fvets.2022.854528.
CrossRef - Li W, Zhang X, Chen R, Li Y, Miao J, Liu G, Lan Y, Chen Y, Cao Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J Ethnopharmacol 2020; 254:112740. doi: 10.1016/j.jep.2020.112740. Epub 2020 Mar 6. PMID: 32151757.
CrossRef - Kumar M, Tomar M, Amarowicz R, Saurabh V, Nair MS, Maheshwari C, Sasi M, Prajapati U, Hasan M, Singh S, Changan S, Prajapat RK, Berwal MK, Satankar V. Guava (Psidium guajava L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Foods 2021; 10(4):752. doi: 10.3390/foods10040752. PMID: 33916183; PMCID: PMC8066327.
CrossRef - Jurić T, Mićić N, Potkonjak A, Milanov D, Dodić J, Trivunović Z, Popović BM. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem 2021; 362:130226. doi: 10.1016/j.foodchem.2021.130226. Epub 2021 Jun 2. PMID: 34118512.
CrossRef - Sankhalkar S, Vernekar V. Quantitative and Qualitative Analysis of Phenolic and Flavonoid Content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacognosy Res 2016; 8(1):16-21. doi:10.4103/0974-8490.171095
CrossRef - Abdin M, Hamed YS, Akhtar HMS, Chen D, Chen G, Wan P, Zeng X. Antioxidant and anti-inflammatory activities of target anthocyanins di-glucosides isolated from Syzygium cumini pulp by high speed counter-current chromatography. J Food Biochem 2020; 44(6):1050-1062. doi: 10.1111/jfbc.13209. Epub 2020 Mar 24. PMID: 32212170.
CrossRef - Jiang TA. Health Benefits of Culinary Herbs and Spices. J AOAC Int 2019; 102(2):395-411. doi: 10.5740/jaoacint.18-0418. Epub 2019 Jan 16. PMID: 30651162.
CrossRef - Khubber S, Hashemifesharaki R, Mohammadi M, Gharibzahedi SMT. Garlic (Allium sativum L.): a potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr J 2020; 19(1):124. doi: 10.1186/s12937-020-00643-8. PMID: 33208167; PMCID: PMC7673072.
CrossRef - Pérez-Gregorio MR, Regueiro J, González-Barreiro C, Rial-Otero R, Simal-Gándara J. Changes in antioxidant flavonoidsduring freeze-drying of red onions and subsequent storage. Food Control 2011, 22:1108–1113.
CrossRef - Chakraborty AJ, Uddin TM, Matin Zidan BMR, Mitra S, Das R, Nainu F, Dhama K, Roy A, Hossain MJ, Khusro A, Emran TB. Allium cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid Based Complement Alternat Med 2022; 2022:4586318. doi: 10.1155/2022/4586318. PMID: 35087593; PMCID: PMC8789449.
CrossRef - Jeon SY, Baek JH, Jeong EJ, Cha YJ. Potential of onion peel extract as a functional ingredient for functional foods. J Life Sci 2012; 22:1207–1213.
CrossRef - Veiga AA, Irioda AC, Mogharbel BF, Bonatto SJR, Souza LM. Quercetin-Rich Extracts from Onions (Allium cepa) Play Potent Cytotoxicity on Adrenocortical Carcinoma Cell Lines, and Quercetin Induces Important Anticancer Properties. Pharmaceuticals (Basel) 2022; 15(6):754. doi: 10.3390/ph15060754. PMID: 35745673; PMCID: PMC9228762.
CrossRef - Mahmoud A, Afifi MM, El Shenawy F, Salem W, Elesawy BH. Syzygium aromaticum Extracts as a Potential Antibacterial Inhibitors against Clinical Isolates of Acinetobacter baumannii: An In-Silico-Supported In-Vitro Study. Antibiotics (Basel) 2021; 10(9):1062. doi: 10.3390/antibiotics10091062. PMID: 34572644; PMCID: PMC8472170.
CrossRef - Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med 2012 ; 7(1):26. doi: 10.1186/1749-8546-7-26. PMID: 23176585; PMCID: PMC3577437.
CrossRef - Hassan W, Noreen H, Rehman S, Gul S, Kamal MA, Kamdem JP, Zaman B, da Rocha JBT. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr Top Med Chem 2017; 17(12):1336-1370. doi: 10.2174/1568026617666170102125648. PMID: 28049396.
CrossRef - Parikh B, Patel VH. Total phenolic content and total antioxidant capacity of common Indian pulses and split pulses. J Food Sci Technol 2018; 55(4):1499-1507. doi: 10.1007/s13197-018-3066-5. Epub 2018 Mar 5. PMID: 29606764; PMCID: PMC5876220.
CrossRef - Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants (Basel) 2017; 6(4):42. doi: 10.3390/plants6040042. PMID: 28937585; PMCID: PMC5750618.
CrossRef - Mabasa XE, Mathomu LM, Madala NE, Musie EM, Sigidi MT. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem Res Int. 2021; 2021:2854217. doi: 10.1155/2021/2854217. PMID: 34621548; PMCID: PMC8492264.
CrossRef - Foss K, Przybyłowicz KE, Sawicki T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum Nutr. 2022; 77(3):383-389. doi: 10.1007/s11130-022-00989-w. Epub 2022 Jul 2. PMID: 35780286; PMCID: PMC9463321.
CrossRef - Kasrati A, Alaoui Jamali C, Bekkouche K, Wohlmuth H, Leach D, Abbad A. Comparative evaluation of antioxidant and insecticidal properties of essential oils from five Moroccan aromatic herbs. J Food Sci Technol 2015; 52(4):2312-9. doi: 10.1007/s13197-014-1284-z. Epub 2014 Feb 16. PMID: 25829614; PMCID: PMC4375224.
CrossRef - Anissa Trad Khodja E, El Hamid Khabtane A, Arhab R, Benouchenne D, Sabri Bensaad M, Bensouici C, Erenler R. assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of leaves extracts. J Tradit Chin Med. 2023; 43(2):252-264. doi: 10.19852/j.cnki.jtcm.20230105.003. PMID: 36994513; PMCID: PMC10012202
- Sivapalan S, Dharmalingam S, Venkatesan V, Angappan M, Ashokkumar V. Phytochemical analysis, anti-inflammatory, antioxidant activity of Calotropis gigantea and its therapeutic applications. J Ethnopharmacol. 2023; 303:115963. doi: 10.1016/j.jep.2022.115963. Epub 2022 Nov 25. PMID: 36442758.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Alpana Joshi2*
and Alpana Joshi2*


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal