Method Development and Validation for the Simultaneous Estimation of Levofloxacin and Cefpodoxime Proxetil by Using Rp-Hplc in Combined Tablet Dosage form
Kole Spandana1, Ch. Rathnakar2, Kole Bhavana2
Department Of Pharmaceutical Analysis and Quality Assurance, Guru Nanak Institute Of Pharmacy, Jawaharlal Nehru Technological University, Hyderabad, Andhra Pradesh, INDIA
DOI : http://dx.doi.org/10.13005/msri/110109
Article Publishing History
Article Received on : 15 Aug 2014
Article Accepted on : 29 Aug 2014
Article Published : 20 Aug 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
An isocratic, reversed phase-liquid-chromatographic method was developed for the quantitative determination of Levofloxacin and Cefpodoxime proxetil in combined-dosage form. Alliance -Waters System with Agilant Zorbax Eclipse XBD-C8, (150mm×4.6; 5µm) column with mobile phase containing water with Ortho phosphoric acid: Methanol in the ratio of (80: 20, v/v) was used. The flow rate was 0.5 ml/min, column temperature was 40°C and effluents were monitored at 270 nm. The retention times of Levofloxacin and Cefpodoxime proxetil were 3.096min and 4.559min, respectively. The correlation co-efficient for Levofloxacin and Cefpodoxime proxetil was found to be 1.0 and 1.0, respectively. The proposed method was validated with respect to linearity, accuracy, precision, specificity, and robustness. Recovery of Levofloxacin and Cefpodoxime proxetil in formulations was found to be in the range of 97-103% and 97-103% respectively confirms the non-interferences of the excipients in the formulation. Due to its simplicity, rapidness and high precision. The method was successfully applied for the estimation of Levofloxacin and Cefpodoxime proxetil in combined dosage form.
KEYWORDS:
RP-HPLC; Levofloxacin and Cefpodoxime proxetil.
Copy the following to cite this article:
Spandana K, Rathnakar Ch., Bhavana K. Method Development and Validation for the Simultaneous Estimation of Levofloxacin and Cefpodoxime Proxetil by Using Rp-Hplc in Combined Tablet Dosage form. Mat.Sci.Res.India;11(1)
|
Copy the following to cite this URL:
Spandana K, Rathnakar Ch., Bhavana K. Method Development and Validation for the Simultaneous Estimation of Levofloxacin and Cefpodoxime Proxetil by Using Rp-Hplc in Combined Tablet Dosage form. Mat.Sci.Res.India;11(1). Available from: http://www.materialsciencejournal.org/?p=433
|
INTRODUCTION
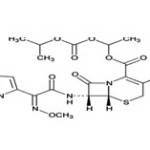
Cefpodoxime proxetil
Cefpodoxime proxetil, (6R,7R)-7-{[(2Z )-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyimino acetyl]amino} -3(methoxy methyl)-8-oxo-5-thia-1 azabicyclo[4.2.0]oct-2-ene-2carboxylic acid is a broad spectrum antibiotic implicated in the treatment of upper respiratory tract and urinary tract infections. The drug is official in Indian Pharmacopoeia and United States Pharmacopeia .The recommended dose of cefpodoxime proxetil is 200 to 400mg per day. The molecular weight of Cefpodoxime Proxetil is 557.6
Structure of Cefpodoxime proxetil
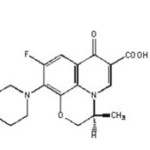
Levofloxacin
Levofloxacin hemi hydrate is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class and is used to treat severe life-threatening bacterial infection or bacterial infection that has failed to respond to other antibiotic classes. IUPAC name is (S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylicacid.
Structure of Levofloxacin
Instrumentation
The separation was carried out on HPLC system with Waters 2695 alliance with binary HPLC pump, Waters 2998 PDA detector, Waters Empower2 software with Agilant Zorbax Eclipse XBD-C8, (150mm×4.6;5µm) column.
Chemicals and Reagents
Cefpodoxime proxetil and Levofloxacin was a gift sample by Dr. Reddy’s Laboratories Ltd., Hyderabad. Methanol of HPLC grade was purchased from E. Merck (India) Ltd., Mumbai. Ortho phosphoric acid of AR grade was obtained from S.D. Fine Chemicals Ltd., Mumbai and mille Q water.
HPLC Conditions
The mobile phase consisting of water (pH adjusted with Ortho phosphoric acid : Methanol (HPLC grade) were filtered through 0.45µ membrane filter before use, degassed and were pumped from the solvent reservoir in the ratio of 80:20v/v was pumped into the column at a flow rate of 0.5ml/min. The column temperature was 40°C. The detection was monitored at 270nm and the run time was 6min. The volume of injection loop was 10µl prior to injection of the drug solution the column was equilibrated for at least 15 min. with the mobile phase flowing through the system.
PREPARATION OF STANDARD SOLUTION
Weigh a quantity of 50mg of Cefpodoxime Proxitel and 40mg of Levofloxacin and transfer it into 100ml clean and dry volumetric flask. Then add mobile phase and sonicate for 30mins and make up the volume with mobile phase and filter through the 0.45µm filter paper. Transfer 5ml of above solution 5ml into 25ml volumetric flask and make up the volume with mobile phase.
PREPARATION OF SAMPLE SOLUTION
Accurately weighed 1037.10mg of sample.Transfer the sample powder into 100ml of volumetric flask added 25ml of mobile phaseand sonicate for 30mins. Then make up the volume with mobile phase and filter through the 0.45µm filter paper. Transfer 5ml of above solution 25 ml volumetric flask and make up the volume with mobile phase.
METHOD VALIDATION
System Suitability Studies
The column efficiency, resolution and peak asymmetry were calculated for the standard solutions (Table1).The values obtained demonstrated the suitability of the system for the analysis of this drug combinations, system suitability parameters may fall within ± 3 % standard deviation range during rountine performance ofthemethod.
Table1: System Suitability Parameters
|
Parameters
|
Levofloxacin
|
Cefpodoxime proxetil
|
|
Correlation Coefficient
|
1
|
1
|
|
Regression Equation
|
y = 46746x
|
y = 38111x
|
|
LOD
|
6.1344
|
5.474
|
|
LOQ
|
20.4479
|
18.246
|
|
Theoretical plates
|
11627
|
8801
|
|
Tailing
|
1.202
|
1.285
|
Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components which may expect to be present. Typically these might include impurities, degradants, matrix, etc
ACCURACY AND PRECISION
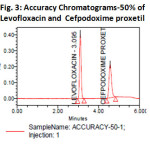
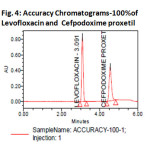
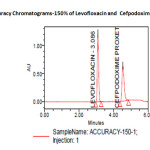
The accuracy of the method was determined by recovery experiments. The recovery studies were carried out six times. The percentage recovery and standard deviation of the percentage recovery were calculated. From the data obtained, added recoveries of standard drugs were found to be accurate (Table-3&4). The precision of the method was demonstrated by inter-day and intra-day variation studies. In the intraday studies, six repeated injections of standard and sample solutions were made and the response factor of drug peaks and percentage RSD were calculated. In the inter-day variation studies, six repeated injections of standard and sample solutions were made for three consecutive days and response factor of drugs peaks and percentage RSD were calculated. the chromatograms of three different levels shown in Fig 3, 4 &5. From the data obtained, the developed RP-HPLC method was found to be precise (Table-2)
inter-day variation studies, six repeated injections of standard and sample solutions were made for three consecutive days and response factor of drugs peaks and percentage RSD were calculated.
Table 2
|
SNO
|
Sample Wt(mg)
|
Area
|
Area
|
%Assya( Levo )
|
%Assya
|
|
(Levo)
|
(Cepo)
|
( Cepo )
|
|
1
|
1037.1
|
4678289
|
3818769
|
100
|
100
|
|
2
|
1037.1
|
4677549
|
3812585
|
100
|
100
|
|
3
|
1037.1
|
4676508
|
3812077
|
100
|
100
|
|
4
|
1037.1
|
4675202
|
3812886
|
100
|
100
|
|
5
|
1037.1
|
4677862
|
3817130
|
100
|
100
|
|
6
|
1037.1
|
4678822
|
3811304
|
100
|
100
|
Table 3: Accuracy for Levofloxacin
|
Spiked Level
|
Sample Weight
|
Sample Area
|
µg/ml added
|
µg/ml found
|
% recovery
|
mean
|
|
50%
|
518.55
|
2336174
|
247.500
|
249.57
|
101
|
101
|
|
50%
|
518.55
|
2339139
|
247.500
|
249.89
|
101
|
|
50%
|
518.55
|
2339510
|
247.500
|
249.93
|
101
|
|
50%
|
518.55
|
2338848
|
247.500
|
249.86
|
101
|
|
50%
|
518.55
|
2332161
|
247.500
|
249.14
|
101
|
|
50%
|
518.55
|
2335555
|
247.500
|
249.51
|
101
|
|
100%
|
1037.10
|
4671180
|
495.000
|
499.02
|
101
|
101
|
|
100%
|
1037.10
|
4670407
|
495.000
|
498.94
|
101
|
|
100%
|
1037.10
|
4679997
|
495.000
|
499.96
|
101
|
|
150%
|
1555.70
|
7018686
|
742.524
|
749.81
|
101
|
101
|
|
150%
|
1555.70
|
7012947
|
742.524
|
749.19
|
101
|
|
150%
|
1555.70
|
7016342
|
742.524
|
749.55
|
101
|
|
150%
|
1555.70
|
7015939
|
742.524
|
749.51
|
101
|
|
150%
|
1555.70
|
7012013
|
742.524
|
749.09
|
101
|
|
150%
|
1555.70
|
7010094
|
742.524
|
748.89
|
101
|
Table 4: Accuracy for Cefpodoxime proxetil
|
Spiked level
|
Sample weight
|
Sample area
|
µg/ml added
|
µg/ml found
|
% recovery
|
mean
|
|
50%
|
518.55
|
1903284
|
198.000
|
199.57
|
101
|
101
|
|
50%
|
518.55
|
1900439
|
198.000
|
199.27
|
101
|
|
50%
|
518.55
|
1909331
|
198.000
|
200.20
|
101
|
|
50%
|
518.55
|
1905283
|
198.000
|
199.78
|
101
|
|
50%
|
518.55
|
1901541
|
198.000
|
199.38
|
101
|
|
50%
|
518.55
|
1906397
|
198.000
|
199.89
|
101
|
|
100%
|
1037.10
|
3818410
|
396.000
|
400.37
|
101
|
101
|
|
100%
|
1037.10
|
3811113
|
396.000
|
399.61
|
101
|
|
100%
|
1037.10
|
3810929
|
396.000
|
399.59
|
101
|
|
150%
|
1555.70
|
5710221
|
594.019
|
598.74
|
101
|
101
|
|
150%
|
1555.70
|
5717081
|
594.019
|
599.46
|
101
|
|
150%
|
1555.70
|
5716768
|
594.019
|
599.42
|
101
|
|
150%
|
1555.70
|
5711949
|
594.019
|
598.92
|
101
|
|
150%
|
1555.70
|
5714713
|
594.019
|
599.21
|
101
|
|
150%
|
1555.70
|
5718874
|
594.019
|
599.65
|
101
|
LINEARITY AND RANGE
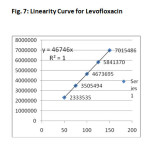
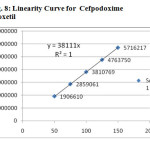
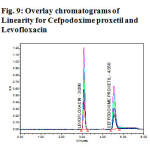
The linearity of the method was determined at five concentration levels. The calibration curve was constructed by plotting response factor against concentration of drugs. The slope and intercept value for calibration curve was Y=46746X(R2=1) for Levofloxacinand Y=38111X(R2=1) for Cefpodoxime proxetil. The results shows that an excellent correlation exists between areas and concentration of drugs within the concentration range indicated above. The overlay chromatograms of Linearity for Levofloxacin and Cefpodoxime proxetil shows in Fig 6 and the results for calibration curves are given in Fig 7&8.
ROBUSTNESS
Robustness of the method was determined by making slight changes in the chromatographic conditions. It was observed that there were no marked changes in the chromatograms, which demonstrated that the RP HPLC method developed, are robust (Table-5&6).
Table5: Robustness for Levofloxacin
| |
SAMPEL NAME
|
INJ
|
NAME
|
RT
|
AREA
|
USP TAILING
|
USP PLATECOUNT
|
|
1
|
TEMP-1
|
1
|
Levofloxacin
|
3.858
|
5842122
|
1.222
|
7582
|
|
2
|
TEMP-2
|
1
|
Levofloxacin
|
3.091
|
4657862
|
1.158
|
9234
|
|
3
|
FLOW-1
|
1
|
Levofloxacin
|
3.092
|
4618822
|
1.097
|
8403
|
|
4
|
FLOW-2
|
1
|
Levofloxacin
|
3.858
|
5842122
|
1.222
|
7582
|
Table6:Robustness for Cefpodoxime proxetil
| |
SAMPEL NAME
|
INJ
|
NAME
|
RT
|
AREA
|
USP TAILING
|
USP PLATECOUNT
|
|
1
|
TEMP-1
|
1
|
Cefpodoxime proxetil
|
5.691
|
4400459
|
1.294
|
7274
|
|
2
|
TEMP-2
|
1
|
Cefpodoxime proxetil
|
4.541
|
3707130
|
1.225
|
7899
|
|
3
|
FLOW-1
|
1
|
Cefpodoxime proxetil
|
4.538
|
3721304
|
1.245
|
7448
|
|
4
|
FLOW-2
|
1
|
Cefpodoxime proxetil
|
5.691
|
4400459
|
1.294
|
7274
|
LOD&LOQ
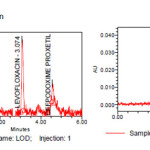
Limit of quantification and detection were predicted by plotting linearity curve for different nominal concentrations of Levofloxacin and Cefpodoxime proxetil. Relative standard deviation (σ) method was applied, the LOQ and LOD values were predicted using following formulas (a) and (b). Precisionwas established at these predicted levels.
(a) LOQ = 10σ / S
(b) LOD = 3.3 σ / S
Where
σ = residual standard deviation of response
S = slope of the calibration
Table 7: LOD and LOQ For Levofloxacin and Cefpodoxime proxetil
|
S.No
|
Sampel Name
|
inj
|
Name
|
RT
|
Area
|
|
1
|
LOD
|
1
|
LEVO
|
3.074
|
73571
|
|
2
|
LOQ
|
1
|
LEVO
|
3.089
|
212865
|
|
1
|
LOD
|
1
|
Cefpo
|
4.564
|
13320
|
|
2
|
LOQ
|
1
|
Cefpo
|
4.578
|
122890
|
RESULTS AND DISCUSSION
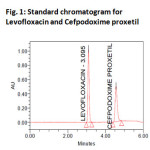
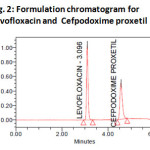
System suitability results were given by table1 and system suitability parameters are retention time, resolution, tailing and plate count were shown uniformity and %RSD was less than 1. So we can say system is suitable for analysis method specificity was concluded by fig:1 and fig:2 those figures are Levofloxacin and Cefpodoxime proxetil standard chromatogram and other one is formulation they were not observed placebo and excipients peaks interference with standard and analytic peak so it proves method is selective. The result given in table 2 says that the method precision passed for both Levofloxacin and Cefpodoxime proxetil studies. The method accuracy was evaluated by recovery studies. Levofloxacin and Cefpodoxime proxetil recovery was founded 100% as per ICH 97%- 103% and also percentage RSD was very low so method is accurate shown in table 3&4. Linearity calibration curve was given below fig: 7&8 and plot the graph three different concentrations versus areas to construct the linear regression equation and to calculate the value of correlation co-efficient.Linear correlation was found to be Y=46746 for Levofloxacin and y = 38111 for Cefpodoxime proxetil Method robustness results were given by table 5&6, LOQ and LOD Results were given by table 7.
CONCLUSION
The proposed HPLC method was found to be simple, precise, accurate and sensitive for the simultaneous estimation of Levofloxacin and Cefpodoxime proxetil pharmaceutical dosage forms. Hence, this method can easily and conveniently adopt for rountine quality control analysis of Levofloxacin and Cefpodoxime proxetil pure and its pharmaceutical dosage forms.
ACKNOWLEDGEMENT
I am thankful to department of Pharmaceutical Analysis and Quality Assurance of Guru Nanak Institute of pharmacy, Jawaharlal Nehru Technological University, Hyderabad, for providing instruments and analytical support.
REFERENCES
- USP 22/NF 17. General chapters<1649>, NF Monographs. Rockville MD. United states Pharmacopoeia convention. 2006; 1710-12.
- ICH, Stability Testing of New Drug Substances and Products, International Conference on Harmonization,IFPMA, Geneva, 2003 Indian Pharmacopoeia-2007. General chapters<p.No.78> Monographs. United states Pharmacopoeia convention. 2006.
- MonikaBakshi,SaranjitSingh., Development of validated stability indicating assay methods-critical review, Journal of Pharmaceutical and Biomedical Analysis., 2002; 28(6); 1011-1040.
CrossRef
- Reynolds, D.W., Facchine, K.L., Mullaney, J.F., Alsante, K.M., Hatajik, T.D., Motto, M.G., Available guidance and best practices For conducting forced degradation studies, Pharm Tech., 2002:48-56.
- FDA Guidance for Industry, Analytical Procedures and Methods Validation (draft guidance), August 2000.
- T. Kumar, A. Chitra, V. Amrithraj, and N. Kumar, “New RP-HPLC method development and validation for estimation of levofloxacin in tablet dosage form,” Journal of Global Trends in Pharmaceutical Sciences, vol. 2, no. 3, pp. 264–276, 2011
- K. Kothekar, J. Balasundaram, A. Khandhar, and R. Mishra, “Quantitative determination of levofloxacin and ambroxol hydrochloride in pharmaceutical dosage form by reversed-phase high performance liquid chromatography,” Eurasian Journal of Analytical Chemistry, vol. 2, no. 1, 2007
CrossRef
- T. Santhoshi, K. Kumar, V. Rao, and A. Ravipati, “Development and validation of RP-HPLC method for simultaneous estimation of levofloxacin and ornidazole in pharmaceutical dosage form,” Journal of Pharmacy Research, vol. 4, no. 11, p. 3864, 2011.
- N. S. Lakka and N. Goswami, “A novel isocratic RP-HPLC method development and validation for estimation of 5HMF in Levofloxacin Hemihydrate intravenous infusion,” International Journal of Research in Pharmaceutical Sciences, vol. 2, no. 1, pp. 45–51, 2011.
- U. Neckel, C. Joukhadar, M. Frossard, W. Jäger, M. Müller, and B. X. Mayer, “Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography,” Analytica Chimica Acta, vol. 463, no. 2, pp. 199–206, 2002.
CrossRef
- J. Mehta, Y. Pancholi, V. Patel, N. Kshatri, and N. Vyas, “Development and validation of a sensitive stability indicating method for quantification of levofloxacin related substances and degradation products in pharmaceutical dosage form,” International Journal of PharmTech Research, vol. 2, no. 3, pp. 1932–1942, 2010.
- M. Green, a Practical Guide to Analyticalmethod validation; Analytical chemistry news and features, May 1, 1996, P. 309A.
- Chen, X, Bates, Morris, K.R. Journal of Pharmaceutical biomedical analysis 2001, 26-63.
- Gombas A., Antal, Szabo, Marton. S., Eros.I,International Journal of pharmaceuticalanalysis 2003, 256,25.
- Kamau F.N, Naugi. J.K, Roets. E. and Chepkwony, H.K. “Isocratic liquid chromatographic method for the analysis ofCefpodoxime proxetil in Bulk sample”. Journal of Chromatographic science. 2002;24(4): 529-533.
CrossRef
- Biljana Nigovic and Branimir Simunic.“Voltammetric assay of Cefpodoxime proxetil in pharmaceutical dosage forms”. Journal of pharm Biomed Anal. 2003;27(6):115-120
Views: 1,867
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal