Synthesis and Characterization of some Transition Metal Complexes with N-Phenyl-N'-[Substituted Phenyl] Thiourea
Mohd. Shadab and Mohammad Aslam
Post Graduate Department of Chemistry G.F. College, Shahjahanpur [U.P], India
DOI : http://dx.doi.org/10.13005/msri/110111
Article Publishing History
Article Received on : 12 Aug 2014
Article Accepted on : 04 Sep 2014
Article Published : 08 Sep 2014
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
A series of thiourea ligand , N-N'- diphenyl thiourea [I] [DPTH], N-phenyl-N'-[2-phenoyl] thiourea [II] [PPTH], N-phenyl-N'-[2-chlorophenyl] thiourea III [PCPTH], N-phenyl-N'- [5-chloro-2-methyl phenyl] thiourea IV [PCMPTH] and N- phenyl -N'-(5-chloro-2-methoxy phenyl) thiourea V (PCMTPTH) and their transition metal complexes of the type ML2 and ML2 Cl2 have been synthesized by reacting phenyl isothiocyanate with substituted aniline and transition metal salts. These newly synthesized ligands and their complexes were characterized by elemental and spectral studies. Based upon these studies it was revealed that in all the cases metal is coordinated through suphur group of thioamide of ligands. In case of nickel complexes, the nickel is coordinated to both oxygen and sulphur. In all the complexes metal is tetra coordinated forming a square planer geometry.
KEYWORDS:
Thiourea derivatives; transition metal complexes; thioamide linkage; square planer geometry
Copy the following to cite this article:
Shadab M, Aslam M. Synthesis and Characterization of some Transition Metal Complexes with N-Phenyl-N'-[Substituted Phenyl] Thiourea. Mat.Sci.Res.India;11(1)
|
Copy the following to cite this URL:
Shadab M, Aslam M. Synthesis and Characterization of some Transition Metal Complexes with N-Phenyl-N'-[Substituted Phenyl] Thiourea. Mat.Sci.Res.India;11(1). Available from: http://www.materialsciencejournal.org/?p=544
|
Introduction
The compounds bearing carbonyl and thiocarbonyl groups are used as potential doner ligand for the preparation of complexes with metals.1,2Among these thiourea derivatives are very versatile ligand. These thiourea ligands and their metal complexes exhibit a wide range of biological activity. They are reported to exhibit antimicrobial, antibacterial, antifungal, antimalarial, antituberclulis and anticancer activities.3-8
Thiourea and its derivatives are versatile ligand which coordinate to form stable compounds. They are able to coordinate to metal containg either as neutral or monoanion or dianion ligand.9-11 They also form a variety of complexes of different symmetries with various metal ions.12-14 These thiourea and their derivatives are not only important in their coordinating ability but are found to exhibit broad spectrum of biological activity. They show wide range of biological activities like analgesic, malaricidal, bactericidal, fungicidal, herbicidal, and insecticidal activities. It was also obserbed that this activity was enhanced by complexing with certain transition metal elements.15-18
In view of the importance of thiourea and their derivatives it was worth interesting to synthesize N-substituted thiourea ligand and their complexes with transition metal elements with the hope that they will produce the compounds of enhanced activity.
Experimental
The chemical phenyl isothiocyanate, aniline, 2-aminophenol, 2-chloro aniline, 5- chloro-2-methyl aniline and 5-chloro-2-methoxy aniline (All Fluka) were used as received.The solvents (All BDH) were used after purification by know method.
The elimental analysis (C,H&N) were carried out by R.S.I.C, C.D.R.I, Lucknow while the sulphur was estimated gravimetrically by known method. The I.R spectra were recorded in the range 4000-200cm-1 on Perkein Elmer 1621 FTIR spectrophotometer.
These results are tabulated in table I and table II
Table –I Elemental analysis of ligands and their metal complexes Found
|
No.
|
Compound
|
Yield
|
C
|
H
|
N
|
S
|
Cl
|
|
1
|
C13 H12 N2 S
|
53
|
67.82
(68.42)
|
4.66
(5.26)
|
11.68
(12.28)
|
13.43
(14.03)
|
|
|
2
|
Mn C26 H24 N4 S2 Cl2
|
56
|
53.01
(53.60)
|
3.52
(4.12)
|
9.01
(9.62)
|
10.39
( 10.99)
|
11.69
(12.19)
|
|
3
|
Ni C26 H24 N4 S2 Cl2
|
58
|
52.75
(53.24)
|
3.36
(4.09)
|
9.02
(9.55)
|
10.25
(10.92)
|
11.72
(12.11)
|
|
4
|
Cu C26 H24 N4 S2 Cl2
|
52
|
52.15
(52.79)
|
3.56
(4.06)
|
8.88
(9.47)
|
10.32
(10.82)
|
11.51
(12.01)
|
|
5
|
C13 H12 N2 OS
|
60
|
63.32
(63.93)
|
4.35
(4.91)
|
10.90
(11.47)
|
12.56
(13.11)
|
|
|
6
|
Ni C26 H22 N4 O2 S2
|
54
|
56.74
(57.24)
|
3.53
(4.03)
|
9.67
(10.27)
|
11.18
(11.74)
|
|
|
7
|
Cu C26 H24 N4 O2 S2 Cl2
|
57
|
49.56
(50.08)
|
3.25
(3.85)
|
8.50
(8.98)
|
9.66
(10.27)
|
10.89
(11.39)
|
|
8
|
C13 H11 N2 S Cl
|
61
|
58.95
(59.42)
|
3.85
(4.19)
|
10.06
(10.66)
|
11.60
(12.19)
|
13.02
(13.52)
|
|
9
|
Mn C26 H22 N4 S2 Cl4
|
64
|
47.32
(47.92)
|
2.77
(3.37)
|
8.10
(8.60)
|
9.33
(9.83)
|
21.31
(21.81)
|
|
10
|
Ni C26 H22 N4 S2 Cl4
|
62
|
47.03
(47.63)
|
2.80
(3.35)
|
8.04
(8.54)
|
9.17
(9.77)
|
21.07
(21.67)
|
|
11
|
Cu C26 H22 N4 S2 Cl4
|
65
|
46.70
(47.27)
|
2.73
(3.33)
|
7.98
(8.48)
|
9.09
(9.69)
|
21.01
(21.51)
|
|
12
|
C14 H13 N2 S Cl
|
63
|
60.25
(60.75)
|
4.10
(4.70)
|
9.62
(10.12)
|
11.07
(11.57)
|
12.23
(12.83)
|
|
13
|
Ni C28 H26 N4 S2 Cl4
|
68
|
48.59
(49.19)
|
3.30
(3.80)
|
7.69
(8.19)
|
8.77
(9.37)
|
20.19
(20.79)
|
|
14
|
Cu C28 H26 N4 S2 Cl4
|
66
|
48.23
(48.83)
|
3.27
(3.77)
|
7.63
(8.13)
|
8.80
(9.30)
|
20.03
(20.63)
|
|
15
|
C14 H13 N2 O S Cl
|
69
|
56.93
(57.43)
|
3.84
(4.44)
|
9.07
(9.57)
|
10.44
(10.94)
|
11.53
(12.13)
|
|
16
|
Ni C28 H26 N4 O2 S2 Cl4
|
67
|
46.49
(46.99)
|
3.03
(3.63)
|
7.23
(7.83)
|
8.35
(8.95)
|
19.26
(19.86)
|
|
17
|
Cu C28 H26 N4 O2 S2 Cl4
|
70
|
46.06
(46.66)
|
3.11
(3.61)
|
7.17
(7.77)
|
8.28
(8.88)
|
19.22
(19.72)
|
Table–2 I.R. Spectra of Ligands and Complexes
|
Compound No.
|
I
|
II
|
III
|
IV
|
-NH
|
-OH
|
M-O
|
M- S
|
M-Cl
|
|
1
|
1540
|
1450
|
990
|
700
|
3320
|
–
|
–
|
–
|
–
|
|
2
|
1566
|
1468
|
1010
|
698
|
3320
|
–
|
–
|
340
|
320
|
|
3
|
1570
|
1462
|
1015
|
698
|
3325
|
–
|
–
|
345
|
322
|
|
4
|
1564
|
1470
|
1010
|
700
|
3320
|
–
|
–
|
345
|
322
|
|
5
|
1548
|
1452
|
995
|
710
|
3325
|
3140
|
–
|
–
|
–
|
|
6
|
1555
|
1466
|
1020
|
705
|
3322
|
–
|
650
|
335
|
–
|
|
7
|
1562
|
1470
|
1022
|
705
|
3325
|
3145
|
|
350
|
325
|
|
8
|
1545
|
1448
|
995
|
700
|
3320
|
–
|
–
|
–
|
–
|
|
9
|
1560
|
1464
|
1020
|
700
|
3320
|
–
|
–
|
350
|
326
|
|
10
|
1570
|
1466
|
1010
|
698
|
3322
|
–
|
–
|
348
|
326
|
|
11
|
1574
|
1458
|
1015
|
698
|
3322
|
–
|
–
|
350
|
328
|
|
12
|
1543
|
1450
|
998
|
695
|
3322
|
–
|
–
|
–
|
–
|
|
13
|
1564
|
1470
|
1008
|
690
|
3330
|
–
|
–
|
360
|
330
|
|
14
|
1570
|
1464
|
1012
|
695
|
3330
|
–
|
–
|
360
|
330
|
|
15
|
1546
|
1445
|
995
|
692
|
3326
|
–
|
–
|
–
|
–
|
|
16
|
1566
|
1460
|
1020
|
690
|
3324
|
–
|
–
|
350
|
330
|
|
17
|
1572
|
1465
|
1015
|
690
|
3326
|
–
|
–
|
355
|
330
|
Preparation of Ligand
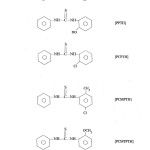
The ligands were prepared by reacting phenyl isothiocyanate with aromatic amines in equimolor ratio.
N-N‘ diphenly thiourea (DPTH)
Phenyl isothiocyanate (0.02 mole, 2.39ml) was added to a solution of Aniline (0.02mole,1.6 ml). The reaction mixture was refluxed for 2-3 hrs. The solution was left for cooling in ice bath, when a white precipitate was obtained, which was filtered, washed with ethanol and dried in vacuum.
N-Phenly-N‘-(2-phenoyl ) thiurea (PPTH)
Phenly isothiocyanate (0.02 mole, 2.39 ml) was added to an ethanoic solution of 2-amino phenol (0.02 mole, 2.19 gm). The reaction mixture was refluxed for 2-3 hrs. The solution was left for cooling in ice bath, when a white precipitate was obtained, which was filtered, washed with ethanol and dried in vacuum.
N- Phenyl-N‘ – (2- chlorophenyl) thiourea (PCPTH)
Phenly isothiocyanate (0.02 mole, 2.39 ml) was added to a solution of 2-chloroaniline (0.02 mole,2.55gm) . The reaction mixture was refluxed for 2-3 hrs. The solution was left for cooling in ice bath, when a precipitate was obtained, which was filtered, washed with ethanol and dried in vacuum.
N-Phenyl-N‘-(5-chloro-2-methyl phenyl) thiourea (PCMPTH)
Phenyl isothiocyanate (0.02 mole, 2.39 ml) was added to a solution of 5 -chloro -2 methyl aniline (0.02 mole, 2.83gm) in ethanol. The reaction mixture was refluxed for 2-3 hrs. The solution was left for cooling in ice bath, when a precipitate was obtained, which filtered, washed with ethanol and dried in vacuum.
N-Phenyl-N‘-(5-chloro-2-methoxy phenyl) thiourea (PCMTPTH)
To a solution of phenyl isothiocyanate (0.02 mole, 2.39 ml) a solution of 5-chloro-2- methoxy aniline (0.02 mole,3.15gm.) in ethanol . The reaction mixture was refluxed for 2-3 hrs. The solution was left for cooling in ice bath, when a precipitate was obtained, which was filtered, washed with ethanol and dried in vacuum.
General procedure for the preparation of complexes
To a solution of ligand in methanol/acetone a solution of metal chloride was added. The reaction mixture was allowed to stirr of about one hour at room temperature when a precipitate of the complex was formed.This reaction mixture was kept in ice bath for some time for complete precipitation.The product so formed was filtered, washed with water and methanol and dried in vacuum.
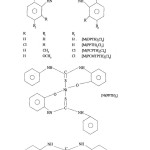
[M(DPTH)2Cl2] M = Mn(II), Ni(II), Cu(II)
These complexes were prepared by reacting solution of ligand and metal salt in 2:1 ratio. The reaction mixture on stirring for about one hour afforded the separation of complexes which were filtered, washed and dried in vacuum.
[Ni (PPTH)2]
To a solution of PPTH in methanol, nickel chloride solution was added and stirred for one hour when greenish coloured product was separated out which was filtered, washed with methanol dried in vacuum.
[Cu(PPTH)2 Cl2]
To a solution of PPTH in acetone, a solution of CuCl2 was added dropwise with constant stirring. After about one hour greyish coloured precipitate was separated out which was filtered washed and dried in vacuum.
[M(PCPTH)2 Cl2] M=Mn(II), Ni(II) Cu(II)
To a solution of PCPTH in methanol, the salt solutions were added. After stirring for about one hour the product separated out which were washed with methanol and dried in vacuum.
[M(PCMPTH)2 Cl2 ]and [M (PCMTPTH)2 Cl2 ] M = Ni(II) Cu(II)
These complexes were prepared by similar procedure as described above with the ligand solution were stirred along with metal salt solution in equimolor ratio.
Results and Discussion
All the ligands, which were prepared by reacting phenyl isothiocyanate with different substituted aromatic amines, found in good yield. These were characterized with elemental analysis and I.R. spectral studies. Their data are compatible with the required product.
The I.R. spectra of the ligand exhibited, in addition to usual absorption bands of phenolic group and their substituents, also the characteristic bands of thioamide group. Compound containing thioamide (HNC=S) group usually give rise to four characteristic band due to different combination of groups. Band I which usually appear at about 1550cm–1 is due to combination of δNH, δCH and υCN vibrations, band II which has combination of υ C=S, υC=N and δCH vibration generally appear in the range 1400-1500 cm–1, band III which usually appear at about 990 cm–1 is mainly due to the combination of υC=N and υC=S vibrations and the band IV usually appearing at about 700 cm–1 is mainly due to contribution of υC=S vibration19, 20,21.
The apperance of these bands in the I.R. spectra clearly assured the reaction of phenyl isothiocyanate to amino group of substituted amine. The absorption bands of primary amine which generally appeare at 3375 cm–1 and 3305 cm–1 found disappeared in ligand and instead a new band at 3320 cm–1 was found for secondary amine clearly indicating the successful reaction of reactants to form ligand22,23.
The treatment of ligands with different metal salt solutions in alcohalic medium afforded metal complexes. The elemental analysis data for these newly synthesized complexes are consistant with proposed molecular stoichiometry. These complexes were further characterized by I.R. spectral studies. The I.R. spectra of all these complexes showed well resolved bands characterized of thioamide group. In addition they also show bands in range 330 – 360 cm–1 clearly assignable to the M-S vibration confirming the complexation of metal through sulphur group.
In the I.R. spectra of all the newly synthesized compounds, all these four bands have been found shifted to higher frequencies. Thioamide band I was found to be shifted to 1540–1585 cm–1 due to coordination of ligands to metal. The band appearing at 1450–1480 cm–1 attributable to thioamide band II was due to coordination of metal to ligands. Similarly the band appeared at about 995–1030 cm–1 was due t o band III and are appeared shifted to high frequency. The band in the range 680–700 cm–1 which was due to band IV found shifted to lower frequency confirming the coordination of metal to ligand through sulphur.
The shifting of these four thioamide bands of the ligands to higher frequencies in complexes indicated that the ligand is coordinated to the metal cation in complexes.
The free ligands in their I.R. spectra exhibited absorption band at 3320 cm–1 attributed
to -NH frequency. In complexes this appeared at about 3320–3340 cm–1 suggesting non involvement of – NH group in coordination22,23.
The I.R. spectrum of ligand II (PPTH) showed a band at 3140 cm–1 assignable to– OH frequency. This band was seen at almost at same level in Cu complex showing non involvement of – OH group in coordination, however in Ni complex it was found disappeared and in addition a new band at 650 cm–1 attributable to M – O frequency was appeared further confirming the coordination of Ni metal through phenolic oxygen24,25,26.
The I.R. spectra of the complexes exhibited bands in the range 330–360 cm–1 which were assigned to M-S vibration. This showed the coordination of metal to the ligand through sulphur of thioamide group.The absorption band at 310 – 340 cm–1 observed in I.R. spectra of the complexes was reasonably assigned to M-Cl vibration.
Acknowledgement
We are thankful to Principal, G.F. College and Head, Deptt. of Chemistry for providing necessary facilities for caring out this work.
References
- Arsalan H.; Kulcu N.; and ForkeU.Transit. Met. Chem. 2003, 28, 816
CrossRef
- Mansuroglu D.S.; Arsalan,H. ; and . Kulcu, N.J. Coord. Chem. 2008, 61, 3134
CrossRef
- Karakus, S.; and Rollas Farmaco S.2002, 577
- Saeed A.; ShaheenU.; Hameed A.; and Naqvi, S.Z.H. J. Fluo. Chem. 2009, 1028
- Rauf, M.K.; Din, I.; Badshah A.; Gielen M.; Ebishara M.; Vos D. and Ahmad, S. J. Inorg. Biochem. 2009 ,1135
CrossRef
- Saeed, S.;Rashid, N.; Jones P.G.;, Ali M. ; and Hussain European R. J. Med. Chem. 2010, 1323
- Isab, A.A.; Nawaz, S.; Saleem ,M.; Altaf, M.; Mahboob, M.M.; Ahmad, A.; and evans ,H.S.Polyhedron. 2010, 1251
- Solamon, V.R.; Haq, W.; Smilkstein, M.; Srivastava, K.; Puri, S.K.; and Katti S.B. European J. Med. Chem. 2010, 4990
CrossRef
- Henderson, W.; Nicholson, B.K.; and Rikasd, C.E.F. Inorg. Chem. Acta. 2001, 320, 101
CrossRef
- Datt, M.S.; Sacht, C.; and Benneth ,R.I.Inorg. Chem. Acta, 2002, 338, 210
CrossRef
- Emen, M.F.; and Kulcu, N.J. Coord.Chem. 2006 ,59, 223
CrossRef
- Holt, S.L.; and Carlin, R.L.J. Am .Chem. soc. 1964, 86, 3017
- Bailey, R.A.; and Peterson Can, T.R.; J. Chem. 1968 ,46, 3119
- Puglisi, C.; and Levitus ,R. J. Inorg. Nucl. Chem.1967, 29, 1069
CrossRef
- Sonar, M.H.; Hirematt ,A.C.; and Sitarmchandra Montash ,A. 1979 ,110, 167
- Xue, S.; Zou ,J.S.; and Yong, H.; Chinese Chem. Lett. 2000, 11(1), 19
- Fengling, C.; Yanrui ,C.; Hongxia, L.; Xiaojon, Y.; Jing, F.; and Yan L, Chinese Chem, Bull, 2006 ,51(18), 2201
- Ke, S.Y.; and Xue, S., Arkivoc, 2006, 10, 63
- Singh, B.; and Thakur ,K.P.; J. Inorg. Nucl. Chem, 1974, 36, 1735
CrossRef
- Abdullah, B.H.; Asian. J. Chem, 2007, 19(5), 3903
- Sharma, J.R.; “Organic Spectroscopy”, S. Chand & Co. 2009
- Narayan, B.; and Galendragad ,M.R.; Turk. J. Chem. 1997 ,21, 71
- El- Ajaily, M.M.; Abdul Saeed, F.A.;and Beugweirif, E-J S.. Chem. 2007, 4(4), 461
- Yalein, M.; Mutluay ,H.; and Cankurtaran ,H.; Turk. J. Chem. 1998 ,22,209
- Silverstein, R.M.; Webster,F.X.; and Kiemle, D.J;. “Spectroscopic identification of Organic Compounds” U.S.A.New York. 2005
- Elzahany, E.A.; Hegab, K.H.; Kholil ,K.H.; and Youssef , N.S.Aust. J. Basic and App. Sci. 2008 ,2(2), 210
Views: 1,626
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal