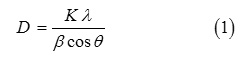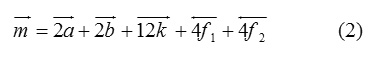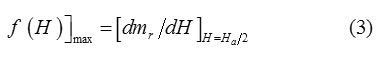I. Bsoul 1*, W. I. Da'as 2
1Physics Department, Al al-Bayt University, Mafraq 130040, Jordan
2Physics Department, Al al-Bayt University, Mafraq 130040, Jordan
DOI : http://dx.doi.org/10.13005/msri/120102
Article Publishing History
Article Received on : 02 May 2015
Article Accepted on : 14 May 2015
Article Published :
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this work, pure and doped with Al (Aluminum) strontium hexaferrite (SrFe12-xAlxO19) were fabricated by solid state reaction method. The Al concentration (x) for the fabricated samples was varied from x=0.0 to x=0.6. Structural and magnetic properties of the fabricated samples were investigated. Analyses of XRD data showed that all samples of high quality, since the measured XRD patterns found to match the standard pattern for single phase of SrFe12O19. The lattice constants a and c were calculated. It was found that the replacement of Fe3+ with Al3+ ions in hexagonal strontium ferrite led to a negligible changes in lattice constant a, whereas the hexagonal lattice constant c found to decrease with increasing the substitution amount of Al. The crystallite size was also determined using the XRD data. It has been found that that Al3+ substitution for Fe3+ does not appreciably disturb the crystallinity of SrFe12-xAlxO19, since the crystallite size for all samples ranged from 83 – 72 nm. The magnetic data showed that doping of strontium with Al leads to a small decrease in saturation magnetization. Also it has been found that the doping of strontium ferrite with Al leads to an increase in the coercivity as a result of increasing of the anisotropy field.
KEYWORDS:
solid state reaction,Strontium ferrite,Coercive field,Anisotropy field
Copy the following to cite this article:
Bsoul I, Da'as W. I. Magnetic properties of SrFe12-xAlxO19. Mat.Sci.Res.India 2015;12(1)
|
Introduction
Strontium ferrite compound (SrFe12O19) belongs to hexaferrite group, which attracts a considerable attention due to their enormous ability to be used as storage media and the fabrication of permanent magnets. Fabrication method plays an important role in determining the magnetic, electrical and some structural properties of the end product. Also the magnetic properties can be controlled by the replacement of magnetic Fe3+ ions by a wide range of magnetic and nonmagnetic cations such as Co, Ni, Zn, Ga, Al, Cr,Ti [1-5] or cations combination such as Mg-Zr, Co-Nd Ni-Sn, Ni-Ru, Zn-Ru, Co-Ti, Ti-Ru [6-15]. These substitutions aim for the development of improved hexaferrite compounds that can be utilized in technological purposes.
Experimental procedures
SrFe12-xAlxO19 powders with x = 0, 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 were prepared by solid state method. Metallic oxides (Fe2O3, and Al2O3) and strontium carbonate (SrCO3) were used to fabricate pure and doped with aluminum strontium ferrite powders. Metallic oxides were weight in appropriate amount then loaded into a hardened stainless steel bowels with ball to powder ratio of 8:1. The milling process was carried out at 250 rpm for 16h. The resulting precursor was annealed at 1100°C for 2 h. XRD data was collected using Philips X’Pert PRO X-ray diffractometer (PW3040/60). The magnetic data were measured at room temperature using Princeton Measurements Corporation vibrating sample magnetometer (VSM). Rietveld refinement was used to refine XDR data using a will known FULLPROF suite package.
Results and Discussion
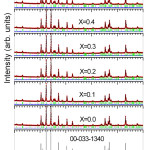
Figure 1 shows the XRD patterns for all samples examined in this work (x = 0.0 to x = 0.6). All XRD patterns were identified by ICDD file (00-033-1340) which belongs to hexagonal strontium ferrite (SrFe12O19) [16]. Since only one phase is present in all samples, we may conclude that the prepared samples are of high quality and the Al3+ ions diffused completely into the lattice of SrFe12O19. Rietveld refinement for all fabricated samples is also shown at Figure 1. Bragg factor (RB) and the goodness of fit quality (c2) are shown in Table 1. The value of c2 for all fitted samples nearly one, which indicates an excellent fit. The structural parameters for all samples are also tabulated in Table 1. The obtained lattice parameters agree well with the previously reported values for hexagonal strontium ferrite [17]. In this work, low doping level (x = 0.1 to x = 0.6) was investigated. XRD data showed that Al3+ substitution for Fe3+ in such low doping level does not affect the hexagonal structure of SrFe12O19. A negligible changes in lattice constant a can be seen in Table 1, whereas the hexagonal lattice constant c decrease with increasing the substitution amount of Al. The decrease of c can be referred to the difference in ionic radius, since the radius of Fe3+ ion, 0.645 Å, is larger than that for Al3+ ion, 0.51 Å. Hence the crystal structure becomes more compact and the unit cell volume decreases as a result of Al3+ substitution. The crystallite size for the prepared samples was calculated according to Scherrer equation [18]:
Figure 1 Refined XRD patterns for SrFe12-xAlxO19 samples along with standard ICDD pattern for SrFe12O19.
Table 1: Bragg factor, χ2, lattice constants a and c, unit cell volume and crystallite size of SrFe12-xAlxO19.
|
x
|
RB
|
χ2
|
a = b (Å)
|
c (Å)
|
V (Å)3
|
D (nm)
|
|
0.0
|
2.69
|
1.103
|
5.8814
|
23.0355
|
690.0658
|
83
|
|
0.1
|
2.08
|
1.066
|
5.8798
|
23.0291
|
689.4998
|
82
|
|
0.2
|
2.47
|
1.072
|
5.8783
|
23.0234
|
688.9736
|
82
|
|
0.3
|
2.33
|
0.9735
|
5.8786
|
23.0302
|
689.2589
|
72
|
|
0.4
|
1.80
|
0.9497
|
5.8767
|
23.0227
|
688.5897
|
74
|
|
0.5
|
1.76
|
0.8768
|
5.8738
|
23.0069
|
687.4166
|
77
|
|
0.6
|
1.62
|
0.8493
|
5.8728
|
23.0031
|
687.0798
|
74
|
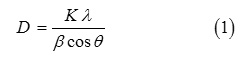
where D is the crystallite size, K Scherrer constant, l X-ray wavelength (1.54056 Å), b full width of half maximum, and q is the peak position in degree. The (114) reflection was selected for the analysis, (after eliminating Kα2 components for more accuracy) since it is the strongest reflection. The results are tabulated in Table 1. From these data we may assume that Al3+ substitution for Fe3+ does not appreciably disturb the crystallinity of SrFe12-xAlxO19, since the crystallite size for all samples ranged from 83 – 72 nm.
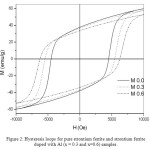
Figure 2 shows hysteresis loops for pure and doped strontium ferrite samples. The effect of Al3+ doping is clearly seen on the coercivity and saturation magnetization. As it was reported the magnetic moment in hexaferrites comes from Fe ions at different interstitial sites and can be given according to the equation [19-21]:
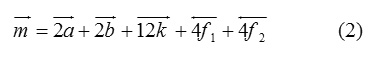
Figure 2: Hysteresis loops for pure strontium ferrite and strontium ferrite doped with Al (x = 0.3 and x=0.6) samples.
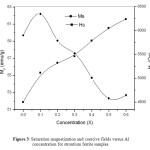
The spin-down sites (4f1 and 4f2) have two Fe ions each, whereas the spin-up sites 2a and 2b have one Fe ion each, and 12k have six Fe ions. Figure 3 shows the saturation magnetization for SrFe12-xAlxO19. For x=0.1 saturation magnetization increases from ~59.7 emu/g to 62.2 emu/g recording 4.2% increase in saturation magnetization. Which indicating that the substitution of Fe ions by non-magnetic Al ions occurs at spin-down sites (4f1, 4f2). For x=0.5 the saturation magnetization decreases to ~52.3 emu/g recording 12.3% drop in saturation magnetization. This drop indicating that the replacement occurs at spin-up sites (2a, 2b, 12k).
Figure 3: Saturation magnetization and coercive fields versus Al concentration of strontium ferrite samples.
Table 2 shows refined sites occupancies for all samples. The atomic number for Fe is 26 and for Al is 13. So site occupancy would be reduced if Fe ions replaced by Al one. According to the data in Table 2, we may conclude that for x=0.1 the replacement of Fe ions occurs most likely at 4f1 spin-down site. Since site occupancy changes from 0.0400 to 0.0387. For x=0.2 to x=0.5 the replacement occurs at 12k spin-up site. Since site occupancy changes from 0.12000 to 0.11583.
Table 2: Refined site occupancy for SrFe12-xAlxO19.
|
Site Occupancy
|
|
Atom
|
Site
|
x=0.0
|
x=0.1
|
x=0.2
|
x=0.3
|
x=0.4
|
x=0.5
|
X=0.6
|
|
Sr
|
2d
|
0.02000
|
0.02000
|
0.02000
|
0.02000
|
0.02000
|
0.02000
|
0.02000
|
|
Fe1
|
2a
|
0.02000
|
0.01996
|
0.01879
|
0.01875
|
0.01817
|
0.01828
|
0.01826
|
|
Fe2
|
2b
|
0.02000
|
0.01819
|
0.01878
|
0.01907
|
0.01876
|
0.01875
|
0.01875
|
|
Fe3
|
4f1
|
0.04000
|
0.03868
|
0.03931
|
0.03972
|
0.03950
|
0.03906
|
0.03898
|
|
Fe4
|
4f2
|
0.04000
|
0.04051
|
0.03982
|
0.04005
|
0.04081
|
0.03925
|
0.04001
|
|
Fe5
|
12k
|
0.12000
|
0.11767
|
0.11700
|
0.11650
|
0.11638
|
0.11583
|
0.11573
|
|
O1
|
4e
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
|
O2
|
4f
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
0.04000
|
|
O3
|
6h
|
0.06000
|
0.06000
|
0.06000
|
0.06000
|
0.06000
|
0.06000
|
0.06000
|
|
O4
|
12k
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
|
O5
|
12k
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
0.12000
|
Figure 3 also shows the coercive fields of SrFe12-xAlxO19 as a function of x. As one might observe the value of the coercivity for pure sample is 4500 Oe, which agrees will with the literature value, since the coercivity of pure SrFe12O19 prepared by different methods was reported in the range (3500 – 5500) Oe [22, 23]. Also it is clear that doping of strontium ferrites with Al leads to significant increase in the coercivity as a result of the Al replacement of Fe3+. The increase of the coercivity can be referred to the increase of the magnetic anisotropy field (Ha) as a result of Al substitution of Fe3+.
We may enhance the above assumption by calculating the effective magnetic anisotropy field for the prepared samples from the maximum of switching field distribution (SFD) according to the equation [24]:
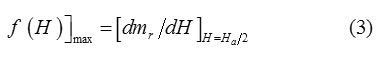
where mr(H) = Mr(H)/Mr(∞) is the reduced isothermal remnant magnetization (IRM), and dmr/dH called switching field distribution (Figure 4). The magnetic anisotropy field (Ha) is equal to 2Hmax.
Figure 4: Reduced isothermal remnant magnetization curves and corresponding SFD for the samples with x = 0.0 and x = 0.6.
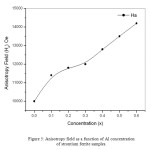
Figure 5 shows magnetic anisotropy field as a function of Al concentration. It is clear that Ha increase monotonically with increasing Al concentration up to x = 0.6, which might be the reason of enhancing the coercivity. It should be noted that, the increase in anisotropy field leads to increase in energy barriers, so the required field to reverse the magnetization become larger, which enhance the coercivity.
Figure 5: Anisotropy field as a function of Al concentration of strontium ferrite samples.
Conclusions
Strontium ferrite powders doped with different concentrations of aluminum (SrFe12–xAlxO19) was prepared by solid state reaction method. Analyses of XRD measurements showed that all samples examined in this work of high quality. The replacement of Fe3+ with Al3+ ions in hexagonal strontium ferrite led to negligible changes in lattice constant a, whereas the hexagonal lattice constant c found to decrease with increasing the substitution amount of Al. Also it has been found that that Al3+ substitution for Fe3+ does not appreciably disturb the crystallinity of SrFe12-xAlxO19, since the crystallite size for all samples ranged from 83 – 72 nm. The magnetic data showed that the doping of strontium with Al leads to a small decrease in saturation magnetization. While the coercivity increases as a result of increasing of the anisotropy field.
References
- El-Sayed S. M., Meaz T. M., Amer M. A., El Shersaby H. A., Physica B, 2013, 426, 137.
CrossRef
- Marino-Castellanos P. A., Moreno-Borges A. C., Orozco-Melgar G., Garcia J. A., Govea-Alcaide E., Physica B, 2011, 406, 3130.
CrossRef
- Slama J., Gruskova A., Papanova M., Kevicka D., Dosoudil R., Jancarik V., Gonzalez A., Mendoza G., J. Magn. Magn. Mater,. 2004, 272-276, 385.
CrossRef
- Awawdeh M., Bsoul I., H. Mahmood S., J. Alloys Compd., 2014, 585, 465.
CrossRef
- Bsoul I., H. Mahmood S., J. Alloys Compd., 2010, 489, 110.
CrossRef
- Sharma M., Kashyap S. C., Gupta H. C., Physica B, 2014, 448, 24.
CrossRef
- Herme C. A., Bercoff P. G., Jacobo S. E., Physica B, 2012, 407, 3105.
CrossRef
- Gonzalez-Angeles A., Mendoza-Suarez G., Gruskova A., Toth I., Jancarik V., Papanova M., Escalante-Garcia J. I., J. Magn. Magn. Mater., 2004, 270, 77.
CrossRef
- Gonzalez-Angeles A., Mendoza-Suarez G., Gruskova A., Lipka J., Papanova M., Slama J., J. Magn. Magn. Mater., 2005, 285, 450.
CrossRef
- Gruskova A., Slama J., Dosoudil R., Kevicka D., Jancarik V., Toth I., J. Magn. Magn. Mater,. 2002, 242–245, 423.
CrossRef
- Bsoul I., Mahmood S. H., Abdel-Fatah Lehlooh, Ahmed Al-Jamel, J. Alloys Compd,. 2013, 551, 490.
CrossRef
- Bsoul I., Mahmood S. H., Abdel-Fatah Lehlooh, J. Alloys Compd., 2010, 498, 157.
CrossRef
- Topal U., Physica B, 2012, 407, 2058.
CrossRef
- Topal U., Bakan H., J. Eur. Ceram. Soc., 2010, 30, 3167.
CrossRef
- Topal U., J. Alloys Compd,. 2008, 320, 331.
- International Centre for Diffraction Data (ICDD), File 00- 033-1340.
- Obradors X., Solans X., Collomb A., Samaras D., Rodriguez J., Pernet M., Font-altaba M., J. Solid State Chem,. 1988, 72, 218.
CrossRef
- Warren B. E., X-Ray Diffraction, Addison-Wesley, Reading, Massachusetts, 1969.
- An S. Y., Shim I. B., Kim C. S., J. Appl. Phys., 2002, 91, 8465.
CrossRef
- Zhou X. Z., Morrish A. H., Li Z. W., Hong Y. K., Ieee Trans. Magn., 1991, 27,4654.
CrossRef
- Williams J. M., Adetunji J., Gregori M., J. Magn. Magn. Mater., 2000, 220, 124.
CrossRef
- Lu H.F., Hong R.Y., Li H.Z., J. Alloys Compd., 2011, 509, 10127.
CrossRef
- Hessien M. M., Rashad M. M., El-Barawy K., J. Magn. Magn. Mater., 2008, 320, 336.
CrossRef
- Pfeiffer H., Schüppel W., Phys. Stat. Sol. (a), 1990, 119, 259.
CrossRef
Views: 935
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal