An Extractive pH Dependent Solid Phase Spectrophotometric (SPS) Determination of Mn (II) and Cu (II) By Using N, N′-Bis(5-(4-Nitrophenyl)Diazenyl)-2-Hydroxybenzylideneamino) Ethylenediimine (NDHBDED) Modified with Alumina
Introduction
First transition series metals have wide spectrum of industrial applications, especially in steel, catalyst, alloy, electroplating, ceramics & petrochemical industries1-3. Some of them play important roles in the biology of micro-organisms, plants and human nutrient. Among heavy elements, Mn (II) and Cu (II) metal ions are problematic in human physiology4-5.
Manganese is widely distributed in earth crust and biological bodies. Though, manganese is one of the essential elements for human health and for other animal’s kingdom as well as for plants6-9. Manganese plays an important role across the life span of all mammals. The role of manganese, as a required co-factor for enzymes and basically, in arginase, which is responsible for urea production in the lever, for superoxide dismutase, which is very important antioxidant enzyme. But its higher level is toxic for human health. It affects the central nervous system. Manganase not only affects the ecosystem, but it also causes erosion of household utensils, bath accessories, clothes and unpleasant taste in food, drinks10-14.
Another heavy metal copper is both vital and toxic for many biological systems. Although trace amount of copper is essential for many biological systems, but its higher concentration is an anthropogenic pollution. It plays a key role in the formation of hemocyanin which is an important respiratory protein. From the standpoint of human health, its role is involved in hemopoiesis and in maintenance of vascular and skeletal integrity and function of the central nervous system. It occurs naturally in most vegetables, meats, and grains. It plays a key role in regulating vital biological processes15-17.
In view from the above standpoints, the separation and determination of Mn ((II) and Cu (II) from different environmental matrices is of great importance. For the determination of both the metal ions at micro level there are several frequently adopted analytical techniques such as electro-thermal atomic absorption spectrometry (ETAAS), AAS, inductively coupled plasma mass spectrometry (ICP-MS), X-ray fluorescence spectrophotometry, spectrofluorometry, and other such technique. Several methods such as liquid-liquid extraction, precipitation, cloud point extraction, co-precipitation and solid phase extraction etc. are used for preconcentration and separation of heavy metals. Out of these above method, the spectrophotometric methods are cheaper and easier to operate and have comparable sensitivity.
A good number of spectrophotometric agents are used for the spectrophotometric determination of the heavy metal ions. These are neutral polymer Amberlite XAD, Amberlite IR-120, activated carbon, octadecyl silica-gel, Dowex IX8, benzophenone, Styrene-divinylbenzene, Pyridine-2,6- dicarboxylic acid, Pyridine-2,6-dimethanol, 2-hydroxy-4-methoxy acetophenone oxime, 4-(2-pyridylazo)resorcinol, malachite green, picolinaldehyde nicotinoyl hydrazone etc18-25. But few of them are used for the extraction and determination of the heavy metal ions26-30. Azo dye Schiff base ligands are important N2O2-donor site containing reagents where some importants heavy metal ions form stable complexes. The metal chelates of these N2O2-containing azo dye Schiff base ligands find wide range applications in biological activities and in analytical chemistry. Herein, we introduced a new azo dye Schiff base ligand N, N′-bis(5-(4-nitrophenyl)diazenyl)-2 hydroxybenzylideneamino) ethylenediamine (NDHBDED) for selective solid phase spectrophotometric (SPS) determination of some heavy metal ions from environmental samples. In this report, we impregnated the azo dye Schiff base ligand with alumina for the complexation with Mn (II) and Cu (II) ions prior to solid phase spectrophotometric (SPS) determination of these metal ions.
Experimental
Reagents and Solutions
High purity reagents were of analytical grade from E. Merck, Germany and other metal salts from BDH, India also were of analytical grade reagent. p-nitroaniline, salicyldehyde and ethylenediamine from Sigma and Aldrich were used for the preparation of the ligand and for the complexation. A neutral solid sorbent alumina was used for the determination of metal ions from Oxford Lab., Mumbai, India. Standard stock solutions of Mn (II) and Cu (II) (1mg/ml) were prepared by dissolving appropriate amount of pure grade chemicals in double distilled deionised water with addition of few drops of conc. HCl. The working solutions of metal ions were prepared by diluting appropriate volumes of stock solution with distilled water. The solution of the chelating agent was prepared in ethanol-methanol mixture.
Instrumentation
The UV-Vis spectra were recorded in ethanol-methanol mixture using Shimadzu Spectrophotometer (UV-1800). A mechanical shaker (BOD-incubator, YONA Mfg. Indian Instruments Manufacture Co., Kol-12) was used throughout the experiments. A labtronic pH meter (Model No-23) equipped with a combined glass-calomel electrode was used to monitor the pH of the solutions.
Synthesis of Azo dye Schiff base ligand (NDHBDED)
The ligand N, N′-bis(5-(4-nitrophenyl)diazenyl)-2-hydroxybenzylideneamino) ethylenediimine (NDHBDED) was synthesized by our previous reported method.
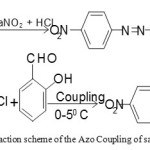
(a) Azo coupling of salicyldehyde
Our proposed diazo compound was synthesized by a reported method. A suspension of p-nitroaniline (2.50 g, 20 mmol) in 30 ml HCl and 10 ml of water was heated from 60-700 C until complete dissolution. The clear solution was diazotized with NaNO2 (2.5 g) dissolved in water (10 ml) at 0-50 C. The cold diazonium solution was added to a solution of salicyldehyde (4.25 ml, 40 mmol) in 50 ml water containing NaOH (1.5 g) and Na2CO3 (10 g) at 00C for 40 minutes. The reaction mixture was stirred during the process. The product was collected and washed with NaCl solution (100ml, 10%). Coupling of the diazonium reagent to the salicyldehyde occurred at the para position to the hydroxyl group. The diazo compound was recrystallized many times from ethyl alcohol and finally dried at 60-700 C for at least 3 hours. Yellow solid, yield 91 %, m.p.165-1700 C, Anal. Calcd. For C13H9N3O4 ; C:57.56, H:3.32, N:15.49, O:23.61%, Found: C:57.60, H:3.31, N:15.45, O:23.6 %. FTIR (KBr cm-1): 1285 (phenolic C-O), 1665 (-CHO group), 1448 (N=N), 1345 (-NO2 group) cm-1 UV-V is:
λ max=390, 545 nm.
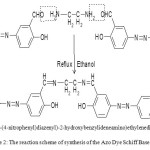
(b) Synthesis of the Azo Dye Schiff Base Ligand (NDHBDED)
For the ligand,N,N′-bis(5-(4-nitrophenyl)diazenyl)-2-hydroxybenzylideneamino)ethylenediimine (NDHBDED), a solution 0.04 mol of previously prepared azo dye and 0.02 mol EDTA in 95 % solution were taken into a 250 ml round bottom flask. Catalytic amount of glacial acetic acid was added to it. The final mixture was allowed to reflux for 3 hours. The product was collected and washed with small amount of ethanol which was soluble in solvents such as ethanol, methanol, DMF and DMSO. The yellow yield was 86 % m.p. 165-1700 C. Anal. Calcd. C28H22N8O6 , C:59.36, H:3.88, N:19.78, O:16.96 %, Found: C:59.50, H:3.91, N:19.81, O:17.00 %. FTIR (KBr cm-1): 3055 (C-H, aromatic), 2890, 2842 (C-H, aliphatic), 1645 (C=N), 1605 (C=C, aromatic), 1420 (N=N), 1275 (C-O, phenolic) cm-1. UV-Vis: λ max= 275,390,460,545 nm.
Scheme 1: The reaction scheme of the Azo Coupling of salicyldehyde.
Scheme 2: The reaction scheme of synthesis of the Azo Dye Schiff Base Ligand.
General Procedure
2 g of the azo dye Schiff base (NDHBDED) chelating reagent was impregnated on the surface of 4.0 g of alumina at the ratio 1:2 in ethanol-methanol mixture and stirred for 30 minutes at room temperature. The resulting composition was left in an oven at 600 C for 48 hours. After drying, the sorbent was ground into powder for metal ions extraction. For the batch method of extraction 300mg of sorbents were used in each 100 ml flasks with 25 ml of 0.01 (M) solutions of Mn (II) and Cu (II) ions and pH was adjusted to 6.5 using HCl (0.1 M) and NaOH (0.1 M) solutions. The mixture of the sorbent matrix and metal ions solutions were shaken vigorously by a mechanical shaker at room temperature for 30 minutes for adsorption of the metal ions onto the adsorbents.
Results and Discussion
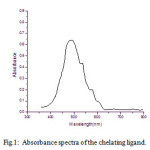
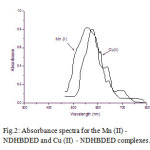
UV-Vis spectra for NDHBDED shows maximum absorbance at λmax 500 nm, but the spectra of the complexes of Mn (II) – NDHBDED and Cu (II) – NDHBDED give maximum absorbance at λ max 590 nm and 570 nm respectively [Fig. 1 & 2]. N, N′-bis(5-(4-nitrophenyl)diazenyl)-2-hydroxybenzylideneamino)ethylenediimine(NDHBDED) forms coloured complexes with Mn (II) and Cu (II) which are stable and their extraction could be quantitative at pH 6.5.
A series of standard solutions containing Mn (II) and Cu (II) in aqueous solution were taken and calibration curve were constructed by plotting absorbance against the amount of Mn (II) and Cu (II) in the conc. range of 10-100 µg and 10-100 µg respectively. The formula of the complexes of Mn (II) and Cu (II) were ascertained by Job’s method and molar ratios methods as 1:1. The analytical parameters of the solid phase spectrophotometric determination of Mn (II) and Cu (II) are given in [Table 1].
Effect of pH
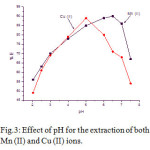
In the quantitative recovery of the metal ions in the solid phase spectrophotometric (SPS) determination pH is a very key factor. The effect of pH for the percentage of extraction of Mn (II) and Cu (II) was investigated at a range 2 to 7.5. The quantitative extraction (> 90 %) found at the pH 6.5 and 5.5 respectively for Mn (II) and Cu (II). Hence, both of the above pH was chosen for the respective metal ions for further studies. Effects of pH on the percentage recovery of the metal ions were shown in Fig.3.
Effect of Temperature
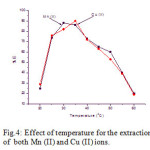
The influence of the temperature on the percentage of extraction of 10 µg/ml of each Mn (II) and Cu (II) was studied in the range of 20-600 C at pH range 5.0 – 6.5. The results showed in Fig.4, that the maximum percentage of sorption of Mn (II) is found at 300 C and Cu (II) is found at 350 C. Hence, for the extraction of both the metal ions, the temperature range was chosen for further studies.
Figure 1: Absorbance spectra of the chelating ligand.
Figure 2: Absorbance spectra for the Mn (II) -NDHBDED and Cu (II) – NDHBDED complexes.
Figure 3: Effect of pH for the extraction of both Mn (II) and Cu (II) ions.
Effect of Amounts of NDHBDED
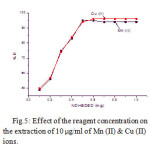
The effect of the reagent concentration impregnated with alumina on the quantitative extraction of metal ions was studied in the range of 0.1 to 1.0 mg. Results show that the extraction of the sorbent was increased on the addition of the reagent. The optimum amount of NDHBDED is at 0.5 mg, where more than 95 % of the metal ions extracted. The results are given in Fig.5.
Effects of metal ions concentration
The percentage extraction of the metal ions in the range of 10-100 µg/ml was studied at a fixed amount of the ligand (0.5 mg) on the solid matrix at pH 6.0. Almost 90 % of both the metal ions were extracted by the sorbent [Table 2].
The stoichiometry of the Mn (II) and Cu (II) – NDHBDED Complexes
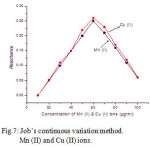
The stoichiometry of the complexes with Mn (II) and Cu (II) ions were determined by Mole-Ratio method and by Job’s method of continuous variation at fixed absorbance of λ max 500 nm.
(a) Mole-Ratio method.
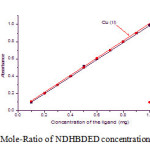
The extraction of the metal ions were studied in the range 0.1 to 1.0 mg of the ligand concentration. The nature of the curves were straight line for both the metal ions which predicting the nature of the complexes are 1:1. The results shown in Fig. 6.
(b) Job’s method of continuous variation.
For fixed volume of the ligand concentration (0.5 mg) with the different concentration of the metal ions 10 – 100 µg/ml were studied. The natures of the curves predicting the nature of the complexs are 1:1. The results are shown in Fig.7.
Figure 4: Effect of temperature for the extraction of both Mn (II) and Cu (II) ions.
Figure 5: Effect of the reagent concentration on the extraction of 10 µg/ml of Mn (II) & Cu (II) ions.
Figure 6: Mole-Ratio of NDHBDED concentration to
Figure 7: Job’s continuous variation method. Mn (II) and Cu (II) ions.
Table 1: Physical and Analytical Characteristics of Mn (II) & Cu (II) – NDHBDED Complexes.
|
Parameters
|
Value
|
|
Mn (II)
|
Cu (II)
|
|
Molar absorbance (λ max )
|
590 nm
|
570 nm
|
|
Limit of Detection (µg/ ml)
|
0.1-1.0
|
0.1-10
|
|
Beer’s law range
|
10-100 µg/ ml
|
10-100 µg/ ml
|
|
Molar absorptivity
|
2.351×10-4 L mol-1 cm-1
|
2..225×10-4 L mol-1 cm-1
|
|
Stability Constant (β)
|
4.49×107
|
3.52X107
|
|
pH
|
6.5
|
5.5
|
|
Temperature
|
300 C
|
350 C
|
|
Standard deviation
|
0.456
|
0.15
|
|
Composition (Metal:Ligand)
|
1:1
|
1:1
|
|
Sandell’s Senstvity
|
0.004 µg/ cm2
|
0.008 µg/ cm2
|
|
Magnetic Moment (µeff)
|
5.89 BM
|
1.723 BM
|
Table 2: Effect of Mn (II) and Cu (II) ions concentration on the percntage extraction of the solid sorbent matrix at pH 6.0 at room temperature.
|
Mn (II)
|
Cu (II)
|
|
Used
(µg/ ml)
|
Extracted
(µg/ ml)
|
% E
|
% RSD
|
Used
(µg/ ml)
|
Extracted
(µg/ ml)
|
% E
|
% RSD
|
|
10
|
7.5
|
75
|
0.12
|
10
|
7.4
|
74
|
0.21
|
|
20
|
15.2
|
76
|
0.14
|
20
|
15.04
|
75.2
|
0.11
|
|
30
|
22.8
|
76
|
0.15
|
30
|
22.89
|
76.3
|
0.09
|
|
40
|
31.8
|
79.5
|
0.09
|
40
|
31.2
|
78
|
0.23
|
|
50
|
41.75
|
83.5
|
0.11
|
50
|
42
|
84
|
0.35
|
|
60
|
51.12
|
85.5
|
0.14
|
60
|
51.6
|
86
|
0.17
|
|
70
|
61.88
|
88.4
|
0.08
|
70
|
60.9
|
87
|
0.27
|
|
80
|
70.88
|
88.6
|
0.06
|
80
|
70.48
|
88.1
|
0.16
|
|
90
|
79.65
|
88.5
|
0.12
|
90
|
79.2
|
88
|
0.2
|
|
100
|
88.5
|
88.5
|
0.12
|
100
|
88.2
|
88.2
|
0.21
|
Effects of Divers ions
The effects of various foreigns ions on the determination of Mn (II) and Cu (II) under optimum conditions was studied. The tolerance of the method of foreigns ions was investigated with solutions containing 1µg/ml each of Mn (II) and Cu (II) from several binary mixtures in a sample volume 50 ml and various amounts of foreigns ions. The determination of both the metal ions was possible in presence of Na+, K+, NH4+, Ca2+, Mg2+, Al3+, Cr3+, Sn2+, Cl–, Br–, CO32-, SO42-, SO32-, CH3COO–, NO3–, NO2–, CN–. Few metal ions, such as Fe (III), Ni (II), Zn (II) and Pb (II) interfere only when present in the same concentration range. The results are presented in Table 3.
Effects of Eluents
Varoius volumes of different eluents were applied for determination of Mn (II) and Cu (II) under optimum conditions. For satisfactory results 3M HNO3 has been used. Various volume of HNO3 was used for extraction of each metal ions and it was found to increase by increasing its volumes. The results is shown in Table 4.
Analytical Application of the proposed method
Optimum pH subjected to the solid phase spectrophotometric (SPS) determination of each metal ions show the results as reported in Table 5. It clear that, the proposed method is suitable for Mn (II) and Cu (II) determination in real samples like tab water.
Table 3: Separation of 1µg/ml each of Mn (II) and Cu (II) from binary mixtures in a sample volume of 50 ml.
|
Ions
|
Amount
(mg)
|
% Recovery
|
Ions
|
Amount
(mg)
|
% Recovery
|
|
Mn (II)
|
Cu (II)
|
Mn (II)
|
Cu (II)
|
|
Na+
|
5
|
98.7
|
99.1
|
Cl–
|
5
|
98.7
|
97
|
|
K+
|
5
|
98.8
|
98.2
|
Br–
|
5
|
97.2
|
98.5
|
|
NH4+
|
5
|
96.5
|
96
|
CO32-
|
5
|
99.2
|
97.6
|
|
Ca2+
|
5
|
97.6
|
97
|
SO42-
|
5
|
96.8
|
98.7
|
|
Mg2+
|
5
|
97.6
|
98.5
|
SO32-
|
5
|
98.3
|
97.2
|
|
Al3+
|
2.5
|
98.1
|
97.6
|
CH3COO–
|
2.5
|
95
|
99.2
|
|
Cr3+
|
2.5
|
97.8
|
98.7
|
NO3–
|
2.5
|
96.3
|
96.8
|
|
Sn2+
|
2.5
|
98
|
97.2
|
NO2–
|
2.5
|
98.7
|
98.3
|
|
Fe3+
|
2.5
|
93
|
94.2
|
CN–
|
2.5
|
95
|
95
|
|
Ni2+
|
2.5
|
89.1
|
90.2
|
–
|
–
|
–
|
–
|
|
Zn2+
|
2.5
|
90
|
90.6
|
–
|
–
|
–
|
–
|
|
Pb2+
|
2.5
|
88.7
|
91
|
–
|
–
|
–
|
–
|
Table 4: Desorption of Mn (II) and Cu (II) by different eluents
|
Eluents
|
% Recovery
|
|
Mn (II)
|
Cu (II)
|
|
1 M HCl
|
75
|
64
|
|
2 M HCl
|
77
|
69
|
|
3 M HCl
|
80
|
73
|
|
1 M HNO3
|
83
|
75
|
|
2 M HNO3
|
86
|
79
|
|
3 M HNO3
|
91
|
90
|
|
3 M CH3 COOH
|
41
|
45
|
|
3 M H2C2O4
|
54
|
51
|
Table 5: Determination of Mn (II) and Cu (II) in real samples and tap water.
|
Samples
|
Certified
Value (μg/g)
|
Proposed
method (μg/g)
|
% RSD
|
|
Soil
|
Mn (II) : 0.45
|
0.64
|
1.13
|
|
Cu (II) : 0.11
|
0.34
|
3.09
|
|
Vegetable
|
Mn (II) : 0.05
|
0.045
|
0.9
|
|
Cu (II) : 0.15
|
0.12
|
0.27
|
|
Tap water
|
Mn (II) : 0.002
|
0.0032
|
1.6
|
|
Cu (II) : 0.015
|
0.002
|
0.13
|
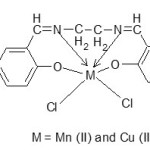
The propposed structure of metal-ligand complexes.
scheme 3
Conclusion
A simple and sensitive solid phase spectrophotometric method for the determination of Mn (II) and Cu (II) ions based on complexation. The method has been applied for the determination of these two metal ions in real samples and tap water. The wide applicability and simplicity of the proposed make it different from other existing methods.
Acknowledgement
The authors are thankful to Dr. Aniruddha Chakraborty for his immense contribution in carrying out this research work. Also, thankful to the Department of Chemistry, University of Burdwan, Burdwan, India for providing the necessary laboratory facilities.
References
- Tavallali H. and Kiani S., Int.J. Chemtech Research, Vol.4, No.3, pp.1182-1186 (2012).
- Rustamov N. Kh., and Abbasova G. G., American J. Anal. Chem., 5, pp.275-280 (2014).
CrossRef
- Moghimi Ali, Australian J. Basic and Applied Sciences, 6(3), 349-355, (2012).
- Salhin Abdussalam, Alhony Aisha Khzam M., Ghani Sulaiman Bin Ab and Salleh Baharuddin, World Applied Sciences Journal, 21 (3), 433-441, (2013).
- Hosseini Mehdi, Dalali Nasser and Nejad Saeid Mohammad, Int. J. of Indust. Chemistry, 3:7 (2013).
- MORI Itsuo, FUJITA Yoshikazu, SAKAGUCHI Kimiko, and KITANO Shoko, The Japan Soc. Of Anal. Chemistry, Vol.31 pp. E239-E242, (1982).
- Chauhan Jayprakash S, Pandya Ajit V, Int. Journal of Eng. Science Invention, 2 (2), (Feb.2013).
- REHMAN F.and MAIRAJ SAMYA, Orient. J. Chem., 28(2), pp. 881-885, (2012).
CrossRef
- Rahman M. F., Chakraborty A.,. Bandyopadhyay S. N, Das T., Golden Research Thoughts, Vol.III, Issue.9, pp.1-6. (March, 2014).
- Anitha C., Sheela C.D., Tharmaraj P.and Shanmugakala R., Int. Journal of Inorg. Chem., Art.ID 436275, (2013).
- Rahman M. F., Chakraborty A. and Das T., IOSR Journal of Applied Chemistry (IOSR JAC), Vol.7, Issue 10, Ver.1, pp.54-69, (Oct.2014).
- Pawar R B, Padgaonkar S B & Sawant A D, Indian J. Chem. Tech., Vol.8, pp.200-203, (2001).
- Hosaini Mehdi & Dalali Nasser, Indian J. Chem. Tech., Vol.19, pp.337-341, (2012).
- Saritha B.and Reddy Prof. Sreenivasulu, IOSR Journal of Applied Chemistry (IOSR JAC), Vol.7, Issue 1, Ver.1, pp.22-26 (Apr.2014).
- Kumar A Praveen, Reddy P Raveendra & Reddy V Krishna, Indian Journal Chemistry,Vol.46A, pp.1625-1629 (Oct. 2007).
- Valencia M.C., Nicolas E. Arana, Capitan-Vallvey L.F., Talanta 49, 915-921, (1999).
CrossRef
- Bosque-Sendra Juan M., Valencia C., Boudra Said, Fresenius J Anal Chem 360, 31-37, (1998).
- Marbaniang D. G., Int. Joutnal of Environmental Protection, 2 (5), pp. 22-26, (2012).
- Siddappa K., Sultana Mayana Nabiya, Int. J. Res. Chem. Environ., 3(2) 78-84, (July 2014).
- MODHAVADIYA V. A., Orient. J., Chem., 28(2), pp. 921-925, (2012).
- Ahmadi Raziyeh Arab and Amani Saeid, Molecules, 17, pp. 6434-6448, (2012)
CrossRef
- Som Sukhen, American J. Pharmacy and Health Research, 2(2), 2321-3647(Online), 2015.
- Kumar Dubey Raj and Nalini Dwivedi, Research J. Of Chem and Environment, 18(3), (March 2014).
- Rahman M. F. & Chakraborty Aniruddha, European Academic Research, 2(3): pp.4195-4203. (June 2014).
- Rahman Md.Faridur, Chakraborty Aniruddha and Das Tanmoy, American J. PharmTech Research, 5(2), (2015).
- Didarul A. Chowdhury, Hoque Md. Ikram ul, Zeenath Fardous, Jordan Journal of Chemistry, Vol.8, No.2, pp. 90-102, (2013).
- Kumar B. Natesh, Venkateshwarlu S.,. Kanchi S, Jyothi N.V.V, International Journal of Analytical and Bioanalytical Chemistry, 3(4), 178-182, ( 2013).
- Kumar Ashok, Sharma Pratibha, Chandel Lal Kumar, Kalal Bhagwan Lal, Kunsagi-Mate Sandor, J Incl phenom Macrocycl Chem, 62, 285-292, (2008).
CrossRef
- LOKHANDE R.S., PATIL V. R., SHEVDE P.P. and LELE S. M., Int. J. Chem. Sci.: 8(2), pp.769-776, (2010).
- Moghimi Ali and Ghammamy Shahriare, Middle-East Journal of Scientific Research, 12, 1331-1338, (2012).
Views: 478
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal