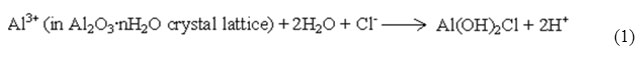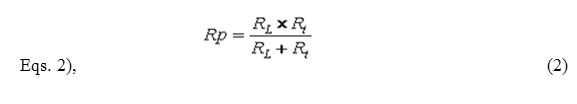Introduction
Particulate reinforced aluminium metal matrix composites (Al MMCs) and other aluminium based materials having huge applications in diverse fields such as light weight automotive structures, forgings for suspension, chassis, as well as advanced automotive components are exposed to a wide variety of corroding environment. Alloys of Al reinforced with ceramic oxides, carbides, nitrides and mineral silicate particulates possess attractive characteristics such as high specific modulus, high specific strength, low thermal expansion coefficient, light weight and low cost [1-3] and superior corrosion resistance.
Studies on Aluminium alloys reinforced with SiC [4], B[5] , Al2O3 [6], TiC [7], and ZrB2[8] report lower corrosion resistance for the composites compared to matrix alloys, owing to galvanic corrosion. On the other hand, Al composites reinforced with garnet [9], albite [10], quartz [11] and glass TiC[12] exhibited higher corrosion resistance compared to their matrix alloys. AlN particles which are highly insulating is reported to increase the susceptibility to pitting attack attributable to microgalvanic coupling between the matrix and the reinforcement and hydrolysis of AlN particles [13]. In the case of TiC-Al 2024 composites the TiC reinforcement is reported to decrease the anodic current density as well as amount and size of the pit [14]. Recent studies on the corrosion resistance of TiB2 [15] particulate reinforced A356 alloy show a marked decrease with increase in TiB2 content is reported by Sun et al. The corrosion resistance of Al-Mg and Al-Cu composites is found to be higher than that of composites reinforced with mica particles [16]. The conflicting results can possibly be explained by differences in fabrication methods and composition which yield dramatically different electrochemical behavior [17].
The observed variation of corrosion resistance in Al MMCs is attributed to chemical or mechanical factors such as composition of the matrix alloy, nature of reinforcing particles, fabrication methods, chemical or mechanical factors such as alloying, segregation, interfacial reactions, oxidized layers, residual stress around reinforced particles in the matrix and galvanic coupling between matrix and reinforcement. The corrosion behaviour of reinforced Al composites investigated [4-16] in acidic, neutral, alkaline and various salt media reveal that Al composites suffer greater localized pitting corrosion in chloride ion environment compared to other media [14-16].
Figure 1: Open circuit potentials for Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %)
composites in 0.5N NaCl medium
Figure 2: Tafel polarisation and Cyclic polarisation plots for Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) composites in 0.5N NaCl medium
Thus since Al MMCs lose their mechanical properties due to corrosion and lead to failure during service, research on the corrosion behaviour is equally important as the studies on fabrication and mechanical behaviour of Al MMCs.
The present investigation involves the study of corrosion behaviour of unreinforced Al-6061 matrix and its TiO2 particulate composites in different electrolytes like NaCl, NaNO3 and Na2SO4. TiO2 having high hardness and modulus is a good reinforcement for aluminium matrix, as it is known to enhance the corrosion resistance of the matrix. The main objective of present work is to establish the role and effectiveness of TiO2 in the corrosion behaviour of Al 6061 composites.
Materials and Methods
Materials Selection
Matrix alloy
Aluminium alloy 6061 used in the present investigation has the composition: Si – 0.6%, Fe – 0.1%, Cu – 0.3%, Mn – 0.02%, Mg – 0.8%, Zn, Cr, Ti – 0.01% each and remaining Al.
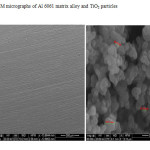
Figure 3: SEM micrographs of Al 6061 matrix alloy and TiO2 particles
Reinforcement
TiO2, A.R Grade was obtained from E-Merck and Sigma Aldrich used as reinforcement in the form of particulates.
Composite preparation
The liquid metallurgy route using vortex technique (Stir Casting) is employed to prepare the composites. The composites were prepared with 2, 4 and 6 percentage by weight of TiO2 using Crucible furnace fitted with a mechanical stirrer. Addition of reinforcement particulates in to the molten Al-6061 (8000C) was carried out by creating a vortex in the melt using a mechanical stainless steel stirrer coated with alumina (to prevent migration of ferrous ions from the stirrer material to the alloy). The stirrer was rotated at a speed of 450 rpm in order to create the necessary vortex. The ceramic particles were pre-heated to 400oC and added in to the vortex of liquid melt at a rate of 20 g/min. The ceramic particulates were of size ~100nm. The composite melt was thoroughly stirred and subsequently degasifiers were added. Castings were produced in permanent moulds in the form of cylindrical rods.
Figure 4: SEM images of Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) Composites taken after polarization studies in 0.5N NaCl medium
Specimen Preparation
Cast material was cut into 20 x 20 mm cylindrical pieces using an abrasive cutting wheel. The matrix alloy was also cast under identical conditions as the composites, for comparison. The samples were successively ground using 240, 320, 400, 600, 800, 1000, 1500 and 2000 grade emery papers and were polished according to standard metallographic techniques and degreased in acetone and dried. For polarisation studies, the samples were cut as cylindrical rods (Diameter 1.128 cm) welded with brass rod (Diameter 4mm) for electrical connection and insulated on the outside using acrylic rubber mould to offer an active flat disc shaped bottom surface of 1cm2. These working electrodes were also polished using 240, 320, 400, 600, 800, 1000, 1500 and 2000 grade emery papers and were polished according to standard metallographic techniques and degreased in acetone and dried.
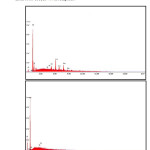
Figure 5: EDX spectra of Al-Fe-Si rich phase and TiO2 phase in Al 6061-TiO2 (6 wt %) composite
Results and Discussion
Open Circuit Potential Measurements
The evolution of the open circuit potential (OCP) was recorded for Al 6061 matrix alloy and its TiO2 composites (2, 4 and 6 wt %) in neutral 0.5N NaCl medium and the plots are shown in Fig.1.The plots and OCP values from the Table 1 reveal that increase towards the positive side as the percentage of TiO2 reinforcement is increased in the composites. The increase in OCP values is in the noble direction from -0.8368 V for Al 6061 matrix alloy to -0.7841 V for reinforced composite (6 wt % TiO2).
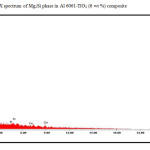
Figure 6: EDX spectrum of Mg2Si phase in Al 6061-TiO2 (6 wt %) composite
The shifting of OCP values to more positive side indicates that the TiO2 particulates in the composites decrease the susceptibility of the composites towards corrosion. The TiO2 reinforcement particles are perceived to act as physical barrier to the corrosion reaction on the Al 6061matrix and thus impart good corrosion resistance to the Al 6061-TiO2 composites.
Table 1: Open circuit potentials of Al 6061matrix alloy and its TiO2 Composites in 0.5N NaCl medium
|
TiO2 content in composites
|
OCP (ECORR) values (in Volts)
|
|
| |
|
0%
2%
4%
6%
|
-0.8368
-0.8198
-0.7948
-0.7841
|
|
TiO2 being an insulator material, reduces the possible formation of microgalvanic regions in the composites compared to the matrix alloy, which accounts for the shift of OCP values to the positive side in the composites. The more noble behaviour of the composites with an increase in the TiO2 content can also be related to the buildup of aluminium oxide at the surface [18, 19, 20].
Figure 7: XRD pattern of Al 6061 matrix alloy and TiO2 particles
Tafel Polarisation Measurements
Typical polarisation curves for Al 6061 matrix alloy and the composites containing 2, 4 and 6 weight % of TiO2 particulates in 0.5N NaCl medium is shown in Fig.2. It is observed from the Tafel plots and Table 2 that both the corrosion current (ICORR) values and the corrosion rates decrease with increase in TiO2 content in the composites. This observation is similar to the findings in SiC reinforced Al composites [4] by Bhat et al.that the composites exhibit reduced corrosion rate compared to the matrix alloy. The decrease in corrosion current with increase in TiO2 content in the Al 6061-composites can be attributed to the reduced anodic areas for dissolution with increasing TiO2 areas. The increased corrosion resistance in composites is believed to be due to the action of TiO2 as physical barrier to the initiation and development of pitting corrosion, thereby modifying the microstructure of matrix.
Figure 8: XRD pattern of Al6061- TiO2 (6 wt %) composite
Table 2: Corrosion current densities (ICORR) and corrosion rates of Al 6061 matrix alloy and its TiO2 composites in 0.5N NaCl medium
|
TiO2 content in composites
|
Corrosion current density, ICORR(Acm-2)
|
Corrosion Rate
(mils per year)
|
|
0%
2%
4%
6%
|
1.163 x 10-6
4.958 x 10-7
3.019 x 10-7
1.368 x 10-7
|
0.4990
0.2414
0.1295
0.0586
|
Other reason for this variation could be the surface modifications like formation of protective layer of mixed oxo, hydroxo and chloro complexes at the interface. The increase in corrosion resistance of composites can also be perceived as due to an increased possibility of formation of magnesium intermetallic precipitates like Mg2Si (ECORR = -1.536 V), Mg2Al3 (ECORR = -1.162 V), Mg5Al8 (ECORR = -1.050 V) at the particulate-matrix interfaces during fabrication.These magnesium intermetallic precipitates acting as sacrificial anodes protect the rest of the matrix, thereby restricting pit formation and its propagation [21, 22, 23]. Wu Jianxin et al. [24] have reported that the corrosion of particle reinforced Al based MMCs is not affected greatly by the presence of SiC particles and attributed the results to probable difference in the conducting nature of reinforcement. They have proposed that the particles of reinforcement do play a secondary role as a physical barrier to initiation and development of pits and also modification in the microstructure of the material leading to reduced rate of corrosion.
Rodriguez [25] has shown that the improved mechanical properties of the particulate reinforced composites are reflected in the improved corrosion resistance of the materials. Extending along the same lines, the increased corrosion resistance of Al 6061-TiO2 composites in our work, point towards improved mechanical properties of these composites. This is supported by the work of Ramesh et al. [26] where increased content of TiO2 in Al 6061 matrix is reported to result in higher hardness and lower wear coefficients. These results evidently point to the fact that TiO2 reinforced composites has higher corrosion resistance as compared to the Al 6061 matrix alloy.
Earlier research workers have reported that halide ions play a significant role in pitting corrosion [27, 28, 29]. Aluminum has good corrosion resistance since its passive film has very low solubility. However, pitting corrosion and other types of localized corrosion in case of aluminum occurs in neutral solutions as a result of the dominant influence of halogen ions, especially Cl− ions. When Cl− ion concentration increases, corrosion potential of aluminium becomes more negative, and aluminum tends to be more sensitive to pitting corrosion. It is believed that competitive adsorption promotes Cl− ions instead of O or oxide on the aluminum surface so that pitting corrosion gets induced. However, the competitive adsorption theory cannot explain satisfactorily the dissolving mechanism of oxide film. According to complexation corrosion theory, compact Al2O3 film is expected to form on the aluminum surface in neutral chloride medium offering good corrosion resistance [30]. The Cl− ions are adsorbed selectively on the crystal lattice of the oxide hydrate film under the effect of electric field. The complexation reaction of Cl− with Al3+ hydrate ions occurs as given in Eqs. (1),
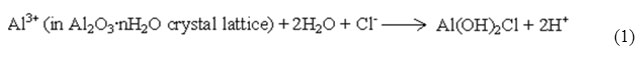
The above reaction results in activation of the aluminum crystallite boundary, so that crystallites break off from the film and become very resolvable and the thickness of the film decreases until local defects are formed. Cl− ions erode the bare surface of Al matrix alloy easily. Once pitting corrosion occurs, the anode current density of small pits (or pit groups) would be much higher than that of a passive surface. Higher the Cl− ion concentration, easier the complexation reaction occurs. That is to say that the dissolving velocity of pit becomes more rapid and therefore the critical pitting potential tends to be more negative with the increase of Cl− ion concentration.
Figure 9: Nyquist plots of the Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) composites in 0.5 N NaCl medium.
The passive film of the aluminum surface is destroyed by the Cl− enrichment sites, which are the centres of pitting corrosion, and then pitting corrosion occurs when the electrode potential reaches the pitting potential. However, the exact mechanism of breakdown of the passive layer by halide ions has not been very clearly understood, especially in the case of aluminium. The aluminium – air or aluminium – water interfaces consist of different solid aluminium oxy hydroxo complexes Al[Ox(OH)y(H2O)z] formed [31, 32]. There is also some evidence for the transformation of the air formed passive layer of amorphous gelatinous alumina in moist air to pseudo-boehmite (AlOOH)4 * H2O, boehmite (AlOOH) and thereafter to bayerite (Al(OH)3) [33, 34]. The discrete structure of the passive layers of different composition can influence the corrosion resistance of Al against chloride induced pitting corrosion due to their different susceptibility to chloride attack. The electrochemical study by Tomcsanyi [35] on Al and its alloys shows that the surface concentration of bonded chloride ions (as AlOCl and Al(OH)2Cl) is larger by two orders of magnitude than that of sulphate ions; chloride ions being bonded chemically in the interface. The highest corrosion rate observed in chloride medium is because chloride ions are more aggressive in pitting [36] due to their adsorbability on passive layer of aluminium compared to SO42− and NO3− ions. The adsorption of chloride ions leads to the breakdown of protective oxide film [37].
Figure 10: Equivalent circuit model used to fit the experimental data of Al 6061 matrix alloy and Al 6061-TiO2 (6 wt %) composite in 0.5N Na Cl medium
Polarisation curves for the Al 6061 matrix alloy and its composites in 0.5N NaNO3 and 0.5N Na2SO4 are identical and exhibit the same trend as observed in 0.5N NaCl medium. Physical examination of the samples reveals significant pitting in chloride medium while pitting is insignificant in both nitrate and sulphate media. It is observed that the corrosion rate is lower in both nitrate and sulphate media, for matrix as well as composites; this can be attributed to their ability to suppress both cathodic and anodic currents [38]. A comparison of the results in all the media indicates that the relative degree of corrosion in different ionic species follows the order Cl− > NO3− > SO42−. The lowest corrosion rate observed in SO42− medium is probably due to the formation of passive aluminium sulphate which is less soluble than other salts formedin the presence of Cl− and NO3− ions.
Cyclic Polarisation Measurements
Cyclic polarisation curves for Al 6061 matrix alloy and the composites containing 2, 4 and 6 weight % of TiO2 particulates in 0.5N NaCl solution are shown in Fig.2. More negative values of the repassivation potentials compared to the pitting potential indicates possible occurrence of pitting. The size of the pitting loop is a rough indication of pitting susceptibility, the larger the loop, the greater the tendency to pit.
From the Fig.2 and Table 3, (Pitting potentials (EPIT), Repassivation potentials (ERP) and ΔE1 (EPIT – ERP) values, it is evident that the pitting potentials of the TiO2 reinforced composites are more positive than those of the matrix alloy. The pitting potential increases in the noble direction with increase in TiO2 contents and ΔE1 values decreases from the matrix alloy to composites. This indicates that the composites are less active and less prone to pitting compared to the matrix alloy. ΔE1 is the direct measure of pitting susceptibility and is used to assess the repassivation behaviour of propagating pits, the ease with which local active sites can be eliminated [39]. The matrix alloy exhibits larger pitting loop as indicated by higher ΔE1 value. In the composites, ΔE1 values decrease with increase in TiO2 content, showing inhibitive action of the TiO2 particulates.
This behavior of the composites in comparison with the matrix confirms the results of our studies viz., OCP and Tafel polarization. Pitting corrosion occurs mainly on the heterogeneities of the metals and alloys in the passive state due to disruption of the passive layer by aggressive species like Cl−[40] and ClO4− ions in the environment [41, 42].
Table 3: Pitting potentials (EPIT), Repassivation Potentials (ERP), ΔE1, ECORR and ΔE2 Values of Al 6061 matrix alloy and its TiO2 composites in 0.5N NaCl medium.
|
TiO2 content in composites
|
EPIT
(V)
|
ERP
(V)
|
ΔE1 = EPIT –ERP
(V)
|
ECORR
(V)
|
ΔE2 = EPIT –ECORR (V)
|
|
0%
|
-0.7002
|
-0.9505
|
0.2503
|
-0.7735
|
0.0733
|
|
2%
|
-0.6981
|
-0.9056
|
0.2075
|
-0.8020
|
0.1039
|
|
4%
|
-0.6959
|
-0.8953
|
0.1994
|
-0.8254
|
0.1295
|
|
6%
|
-0.6938
|
-0.8718
|
0.1780
|
-0.8397
|
0.1459
|
The other factors influencing corrosion in the composites include porosity of the material, segregation of alloying elements at the matrix-reinforcement interface, presence of interfacial reaction product, high dislocation density around the reinforcement phase and voids at the interface [14, 43]. The presence of a less conductive TiO2 phase does not provide any path for electron exchange during oxygen reduction and drives the anodic reaction at a lower rate in the composites as compared to matrix alloy.
ΔE2 valueisa measure of the width of the passive region in the polarization curves and provides information about the resistance of materials to corrosion. Higher the ΔE2 value, greater is the corrosion resistance of composites [44]. The ΔE2 value of 73.3 mV for Al 6061 matrix alloy is found to increase to 145.9 mV for Al-6 wt% TiO2 composite. This clearly shows that the addition of noble insulator TiO2 to Al 6061 matrix significantly increases the passive range from the unreinforced matrix alloy to the reinforced composites. The presence of insulating TiO2 particles can influence the corrosion behaviour of Al 6061-TiO2 composites in two ways.
First the presence of inert particles could physically inhibit the progression by forcing the process of corrosion to move around the particles. As corrosion attack gets deeper into the specimen, it would become more difficult due to longer diffusion paths of the corrosive species. Secondly, the presence of the reinforcing TiO2 particles would result in a finer microstructure than that of an unreinforced matrix alloy, by physical inhibition of normal dendritic growth between the particles, which in turn results in reduced micro segregation or inhomogeneities that may contribute to localised corrosion [45, 46, 47].
It is generally agreed that insulating particulates like TiO2 can influence corrosion, modifying the microstructure of the MMC by altering the size and distribution of intermetallic phases or introducing residual stresses between reinforcement and matrix. The presence of TiO2 particles does influence passive current density and pitting potential of aluminium, to an extent such that the pitting resistance of passive film on aluminium gets significantly affected [38]. Generally the susceptibility to pit initiation is similar for composites and unreinforced alloys; however, the rate of pit propagation is observed [48] to be slightly lower for composites contrary to the work of Aylor et al.
Scanning Electron Microscopy
Scanning electron microscopy images of Al 6061 matrix alloy before corrosion test and that of reinforcement TiO2 particles are shown in Fig.3. The figure shows that the ceramic TiO2 particles are approximately equiaxed with irregular shape and sharp corners although spherical reinforcements are also observed [49, 50]. The particle size of reinforcement TiO2 particulates is about 100 nm as observed in the SEM image. The SEM images of Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) composites taken after the polarization studies in decinormal chloride medium, after the usual pretreatment, are presented in Fig.4.
A comparison of the scanning electron microscopy images of the Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) composites after the polarization studies clearly indicate the severe surface deterioration due to pitting corrosion. The SEM images of corroded samples reveal severe pitting and crack development in Al 6061 matrix alloy than the reinforced composites. A lesser degree of surface deterioration in the case of composites indicates the higher corrosion resistance of composites than matrix alloy
The micrographs of matrix alloy and the composites reveal a complete deterioration of smoothness of surface suggesting the penetration of chloride ions in to the material surface forming corrosion spot. A comparison of SEM micrographs of the samples after polarization studies also suggests morphological modifications probably due to the reaction of Mg2Si phase. Corrosion products resulting from the reaction of Mg2Si prevent further dissolution of the particle and reduce the effect of galvanic coupling with the matrix, thus improving corrosion resistance of composites [51]. In general, Mg2Si phase acts as anode with respect to matrix alloy and forms a galvanic couple leading to pitting corrosion. But Mg2Si is also likely to form oxides like MgO and SiO2, which offer protection to the Mg2Si particles and thereby decrease the number of regions of galvanic coupling between Mg2Si and matrix [52].
Energy Dispersive X-ray Analysis
The EDX spectrum shows the presence of various elements in the TiO2 reinforced Al 6061 surface. The EDX analysis of the two regions (i) the intermetallic phases and (ii) the TiO2 rich phase in the EDX spectrum are presented in Fig.5 and Fig.6. It is observed that the matrix alloy and composites contain the Al-Fe-Si rich and Mg2Si intermetallic phases.
The presence of more intense peaks for Al, Mg, Fe and Si as shown in Fig.5 strongly indicates the existence of Al-Fe-Si rich intermetallic phases like Fe2SiAl8 and Mg2Si. These intermetallic phases being conductive act anodically with respect to Al 6061 matrix alloy and become the preferable sites for initial localised attack thus causing pits [53]. The surface morphological studies around the pits reveal that the corrosion is concentrated around the intermetallics but not around the insulator TiO2 particles which is confirmed by EDX analysis of pits. The presence of Fe and Si suppresses the cathodic reaction rate of the aluminium based intermetallics rich in Fe.
XRD Studies
The XRD patterns of Al 6061 matrix alloy, TiO2 particulates and that of composite (6 wt% TiO2 reinforced) are given in Fig.7 and Fig.8 respectively. The XRD spectra of the composite clearly exhibits the major 2θ peaks of Al 6061 matrix (at 38.290, 44.570 and 64.920) and TiO2 (25.520, 48.220 and 54.070) indicating the incorporation of the reinforcement particulates in the matrix alloy.
EIS Studies
The EIS plots and data which follow reveal that the impedance curve consists of a large capacitive loop at high frequencies (HF) and a small inductive one at low frequencies. Nyquist plots of the Al matrix alloy and its TiO2 composites in 0.5 N NaCl solution is given in Fig.9. The HF capacitive loop is attributed to the presence of a protective oxide film at the surfaces of composites [54, 55]. The HF loop could be assigned to the relaxation process in the hydrated aluminium oxide film and its dielectric properties. The oxide film is considered to be a parallel circuit of a resistor corresponding to the ionic conduction in the oxide film and a capacitor due to its dielectric properties.
The inductive loop observed at low frequencies may be attributed to the relaxation process in adsorbed species OH−ads present on the surface of the samples. The numerical values of the polarisation resistance Rp and the double layer capacitance Cdl were determined by analysis of the complex plane impedance plots and the equivalent circuit model by using a computer program. The charge transfer controlled type corrosion process has been confirmed by the presence of semicircles in the impedance diagrams [56, 57]. Based on the analysis of the impedance spectra, an equivalent circuit model R[QR[LR]] is being proposed for both the matrix alloy and composites (Fig.10), after simulating their electrochemical behaviour. The equivalent circuit consists of a constant phase element (CPE) Q in parallel with the parallel resistors Rt (charge transfer resistance) and RL (inductance resistance) and the latter is in series with the inductor L. In this case the polarisation resistance can be calculated using the
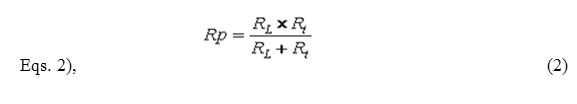
The electrochemical parameters of Al 6061 matrix alloy and its composites obtained from EIS studies are given in Table 4. The measured value of polarization resistance, Rp increases while the constant phase element, CPE value decreases with increase in TiO2 content. Hence our results show that the corrosion rate decreases with increase in the content of TiO2 reinforcement.
Table 4: Electrochemical parameters for Al 6061 matrix alloy and its TiO2 (2, 4 and 6 wt %) composites in 0.5 N NaCl medium.
|
TiO2 content in composites
|
Rs
(ohm)
|
Rp
(ohm)
|
CPE
(F)
|
Corrosion rate
(mpy)
|
|
0%
|
2.420
|
9800
|
2.12 x 10-5
|
0.4990
|
|
2%
|
2.829
|
12200
|
1.49 x 10-5
|
0.2414
|
|
4%
|
3.769
|
22692
|
7.70 x 10-6
|
0.1295
|
|
6%
|
4.008
|
26496
|
5.97 x 10-6
|
0.0586
|
It can be observed from the Nyquist plots, that the radius of the capacitive loops above the real axis increases with increase in TiO2 content for composites. Our results indicate that the polarisation resistance Rp value increases with increase in TiO2 content in composites confirming improved corrosion resistance in composites. These results compliment potentiodynamic polarisation studies.
Conclusions
The corrosion behaviour of unreinforced Al 6061 matrix alloy and its composites reinforced with TiO2 (2, 4 and 6 wt %) were studied in 0.5N NaCl medium. The observations of these studies lead to the following conclusions,
- With increase in the reinforcement content in the composites, the OCP values increase in the noble direction indicating higher corrosion resistance imparted by the TiO2 particulates.
- In each case, it was observed that the corrosion potential ECORR values shift towards negative direction with increase in the concentration of the chloride medium, probably due to the breakdown of stable oxide film on Al matrix as a result of increased adsorption of chloride ions on the Al surface or its oxide layer.
- Corrosion rates of the matrix alloy and the composites were calculated from the Tafel polarization curves, which show a decrease with increase in the content of the reinforcement. The increased corrosion resistance in composites is believed to be due to reinforcement particulates modifying the microstructure of matrix and also acting as physical barrier to the initiation and development of pitting corrosion.
- Pitting potentials EPIT are found to increase in the anodic direction from the matrix alloy to reinforced composites while the pitting loop size (ΔE1 = EPIT -EPP) decreases with increasing content of reinforcement, confirming the increased corrosion resistance due to reinforcement in Al 6061 matrix alloy.
- A comparison of the results from all the media indicates that the relative degree of corrosion in different ionic species follows the order Cl− > NO3− > SO42−. The lowest corrosion rate observed in SO42− medium is due to the formation of passive aluminium sulphates which are less soluble than those salts formed in presence of Cl− and NO3− ions.
- The XRD and EDX analysis of all the composites confirm the positive inclusion of the reinforcement particulates in the matrix alloy and also the presence of intermetallic phases like Mg2Si, Al3Fe and Al4C3 formed at matrix-reinforcement interface.
- SEM pictures of each of the composites and the unreinforced alloy clearly reveal severe deterioration of the surface of both matrix alloy and its composites of all the reinforcements. However there is a decrease in the extent of pit formation in the composites as compared to the matrix alloy corroborating the results of the corrosion studies.
- The Nyquist plots of the matrix alloy and the composites in NaCl medium show semicircles indicating that the corrosion process is mainly charge transfer controlled. The measured value of polarization resistance Rp increases while the CPE value decreases with increase in content of the reinforcement in all the cases.
Acknowledgements
The authors are grateful to Dr. Ambedkar Institute of Technology, Bangalore, India for cooperating to carry out our research work and Indian Institute of Science, Bangalore, India for providing instrumentation facility.
References
- J E Allison and G S Cole., J. Met. 45 (1993) 19-24.
- C G E Mangin, J A Isaacs, J P Clark, J. Met. 48 (1996) 49-51.
- H G Kang, D L Zhang, B Cantor, J. Microstrc. 169 (1993) 239-245.
- M S N Bhat, M K Surappa, Sudhaker Nayak, J. Mat. Sci. 26 (1991) 4991-4996
CrossRef
- S L Pohlman, Corrosion. 34 (1978) 156-159.
CrossRef
- P P Trzaskoma, E McCafferty, C R Crowe, J. Electrochem. Soc. 130 (1983) 1804-1809.
CrossRef
- Rahul Mitra, Yashwanth R.Mahajan, Defense Sci. J. 43 (1993) 397-418.
CrossRef
- J B Fogangolo, M H Robert, Ruiz-Navas, J M Torralba, J. Mat. Sci. 39 (2004) 127-132.
- K H W Seah, M Krishna, V T Vijayalashmi, J Uchil, Corros. Sc. 44 (2002) 917-925.
CrossRef
- S C Sharma, Corros. Sci. 43 (2001) 1877-1889.
CrossRef
- K H W.Seah, B.M.Sathish, Mater.Design. 17 (1996) 245-250.
CrossRef
- H C Ananda Murthy , Somit Kumar Singh, Adv. Mater. Lett. 6(7) (2015) 633-640
- Zhang Sheng Liu, Wu, Bian Tao, Gu Ming Yuan, J. Mat. Sci. 42(14) (2007) 5736-5741.
CrossRef
- A. Albiter, A.Contreras, M.Salazar, G.J.Gonzalez, Rodriguez, J. Appl. Electrochem., 36 (2006) 303-308.
CrossRef
- H.H.Sun, D.Chen, X.F.Li, N.H.Ma, H.W.Wang, Mater. Corros., 60 (2009) 419-423.
CrossRef
- D.Nath, T.K.G.Namboodhiri, Corros. Sci. 29 (1985) 1215-1229.
CrossRef
- D. G. Kolman, D.P.Butt, J. Electrochem. Soc. 144 (1997) 3785-3791.
CrossRef
- A.M.Shams El Din, N.J.Paul, Thin solid films. 189 (1990) 205-216.
CrossRef
- J. M. Abd El Kader, A.M. Shams El Din, Br. Corros. J. 14 (1979) 40-45.
CrossRef
- A. M. Shams El Din, A.A. Hammound, Thin solid films. 167 (1988) 269-280.
CrossRef
- N.N.Birbilis, R.G.Buchheit, J. Electrochem. Soc. 152 (4) (2005) 140-151.
CrossRef
- R.G. Buchheit, J. Electrochem. Soc. 142 (1995) 3994-3996.
CrossRef
- KHW Seah, M Krishna, V T Vijayalakshmi, J Uchil, Corrosion. 44 (2002) 761-772.
CrossRef
- W.Jiaxian, W.Liu, P.X.Li, R.Wu, J. Mat. Sci. Lett. 12 (1993) 1500-1501.
- E .L. Rodreguez, J. Mat. Sci. Lett. 6 (1987) 718-720.
CrossRef
- C.S.Ramesh, A.R.Anwar Khan, N. Ravi Kumar, P. Savanprabhu, Wear. 259 (2005) 602-608.
CrossRef
- T.P.Hoar, W.R.Jacob, Nature. 216 (1967) 1299-1301.
CrossRef
- T.E.Pou, O.J, Murphy, V.Young, O.M.Bokris. J. Electrochem. Soc. 131 (1984) 1243-1251.
CrossRef
- N.Sato, Corros. Sci. 27 (1987) 421-433.
CrossRef
- Cheng-Hao Liang Wei Zhang, J. Chin. Chem. Soc. 53 (2006) 313-318.
CrossRef
- A. Berzins, R.T.Lowson, K.J.Mirans, Aust. J. Chem. 30 (1977) 1891.
CrossRef
- W.C.Moshier, G.D.Davis, J.S.Ahearn, Corros. Sci. 27 (1987) 785-801.
CrossRef
- K.Wefers, G.M.Bell, Alcoa Technical paper No. 19 (1972) 1-47.
- T.Okada, Corros. Sci. 26 (1986) 839-849.
CrossRef
- Tomcsanyi L, Varga K, Bartik I, Horanyi G, Maleczki E, Electrochim. Acta 34 (1989) 855-859.
CrossRef
- H.C. Ananda Murthy, V. Bheema Raju and C. Shivakumara, Bull. Mater. Sci. 36 (2013) 1057-1066.
CrossRef
- M. A. Paez, O. Bustos, G. E. Thompson, P. Skeldon, K. Shimizu, G. C. Wood, J.
CrossRef
- Electrochem. Soc. 147 (2000) 1015-1020.
- P.C.R. Nunes, L.V.Ramanathan, Corros. sci. 51 (8) (1995) 610-617.
CrossRef
- J.Bienias, M.Walczak, B.Surowska, J.Sobczak, J. Optoeln. and Adv. Mat. 5 (2003) 493-502.
- J.Datta, B.Samantha, A.Jana, S.Sinha, C.Bhattacharya, S.Bandyopadhyay, Corros. Sci. 50 (2008) 2658-2668.
CrossRef
- Mohammed A. Amin, Sayed S. Abd El Rehim, S.O.Moussa, Abdallah S.Ellithy, Electrochim. Acta. 53 (2008) 5644-5652.
- G.Wranglen, WNT Warszawa, (1985).
- R.L.Deuis, L.Green, C.Subramaniyan, J.M.Yellup, Corrosion 53 (1997) 880-890.
CrossRef
- S.L.Winkler, M.P.Ryan, H.M.Flower, Corros. Sci. 46 (2004) 893-902.
CrossRef
- A. Mortensen, M.N.Gungor, J.A.Cornie, M.C.Flemings, J. Met. 32 (1986) 30-35.
- A.Mortensen, V.Michuad, Metall. Trans. A 21 (1990) 2069-2072.
- A. Mortensen, J.A.Cornie, M.C.Flemings, Metall. Trans. A 19, (1988) 709-712.
CrossRef
- D.M.Aylor, D.Taylor, Corrosion of Metal Matrix Composites in ASM Handbook, Vol. 13, Corrosion, J.R Davis Ed., ASM International, Materials park, OH (1999) 859-863.
- K.Mahadevan, K.Raghukandan, B.C.Pai, U.T.S.Pillai, Ind. J. Engg. and Mat. Sci. 14 (2007) 277-281.
- W.A. Badawy, F.M, Al-Kharafi, A.S. El-Azab, Corros. Sci. 41 (1999) 709-727.
CrossRef
- R. Escalera-Lozano, M.A. Pech-Canul, M.I. Pech-Canul and P.Quintana, Mater. Corros. 60 (2009) 683-689.
CrossRef
- R.Escalera-Lozano, M.I. Pech-Canul, M.A. Pech-Canul, M.Montoya- Davila, A.Uribe-Salas, The open corrosion journal, 3 (2010) 73-79.
CrossRef
- L.H.Hihara, T.S.Devarajan, Hongbo Ding, G.A. Hawthorn, Tri-Service corrosion conference, (2005). C.M.A.Brett, Corros. Sci. 33 (1992) 203-210.
- C.M.A.Brett, J. Appl. Electrochem. 20 (1990) 1000-1003.
CrossRef
- G. Trabanelli, C.Montecelli, V.Grassi, A.Frignani, J. Cem. Concr. Res. 35 (2005) 1804-1813.
CrossRef
- Irfan aziz, Qi Zhng, Int. J. Phy. B 23 (2009) 1497-1515.
Views: 1,224
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal