Synthesis of Hydroxyapatite and Zno Nanoparticles Via Different Routes and Its Comparative Analysis
P. Mahapatra1, Shreya Kumari1, Simran1, Shruti Sharma3, K. Gaurav2,N. Kumari2, Parameshwar Kommu1,
P. Prabhakar1 and A. S.Bhattacharyya1, 2*
1Centre for Nanotechnology,Central University of Jharkhand, Ranchi – 835 205, India,
2 Centre of Excellence in Green and Efficient Energy Technology (CoE-GEET), Central University of Jharkhand, Ranchi – 835 205, India,
3Centre for Water Engineering and Management, Central University of Jharkhand, Ranchi – 835 205, India,
Corresponding Email: 2006asb@gmail.com
DOI : http://dx.doi.org/10.13005/msri/130102
Article Publishing History
Article Received on : 29 May 2016
Article Accepted on : 11 Jun 2016
Article Published : 09 Jun 2016
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Hydroxyapatite (HAp) was prepared from egg shells by various routes using hexane and acetic acid followed by heat treatment. HAp has a wide application in water treatment by removal of metal ions. XRD of the samples showed use of acetic acid followed by high temperature sintering leads to formation crystalline phases of HAp. Strong evidence of CaCO3 in calcite phase was obtained in other samples. Zinc oxide nanoparticles have also been synthesized by different methods such as sol-gel, co- precipitate and green synthesis. The effect of different synthesis methods were investigated using X-Ray Diffraction (XRD). The structural properties of nanoparticles including particle size were calculated from XRD data. The XRD reveals that the prepared ZnO samples were highly crystalline, having wurtzite crystal structure. The comparative analysis shows variations in particle size with different synthesis methods.
KEYWORDS:
Hydroxyapatite; ZnO; sol-gel; co- precipitation; green synthesis; XRD
Copy the following to cite this article:
Mahapatra P, Kumari S, Simran, Sharma S, Gaurav K, Kumari N, Kommu P, Prabhakar P, Bhattacharyya A.S. Synthesis of Hydroxyapatite and Zno Nanoparticles Via Different Routes and Its Comparative Analysis. Mat.Sci.Res.India;13(1)
|
Copy the following to cite this URL:
Mahapatra P, Kumari S, Simran, Sharma S, Gaurav K, Kumari N, Kommu P, Prabhakar P, Bhattacharyya A.S. Synthesis of Hydroxyapatite and Zno Nanoparticles Via Different Routes and Its Comparative Analysis. Mat.Sci.Res.India;13(1). Available from: http://www.materialsciencejournal.org/?p=3905
|
Introduction
Hydroxyapetite (HAp) having formula Ca10 (PO4)6(OH)2 have a wide application in water treatment. HAp based filter are used for removal of heavy metal ions from aqueous solutions. 1 HAp – Chitosan nanocomposites synthesized by sol-gel method have been used for the remocal of Cd+2 ions in water treatment.2 HAp has been found in fish bone. Porous structure and crystalline phases of HAp were obtained when heated at 1000 oC. It showed potential in removal of Cr ions from natural river.3 3D ordered HAp hollow microspheres were synthesized from soluble bio polymer like polyaspartic acid where initial formation followed by transformation of calcium phosphate spheres were reported.4 Sintering of HAp and Silica composite nano powders have led to formation of porous ceramic filters. The water permeated through them was of drinking water quality.5 Ag3PO4/HAP composites were synthesized facilely via in-situ precipitation of Ag3PO4 on the pre-existing HAP nanowires which can be used as water treatment material.6 Egg shells have been used to synthesize Hap. The egg shells are three layered structure: cutile, spongeous layer and lamellar layer. It consists mostly of Calcium carbonate and small amounts of Calcium phosphate magnesium carbonate and some organic matter. Hydroxyapetite (HAp) are prepared from egg shells in phosphate solution at elevated temperature.7
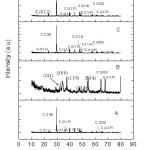
The surfaces of the raw eggshells were mechanically cleaned in order to remove the internal crust lining in the shell. Afterwards the eggshells were washed using tap water for about 15 times. The washed eggshells were then subjected to constant stirring for about 15 – 30 minutes .The sample was then placed in an oven at 100˚C for constant heating for about 6hours.The dried sample (15.4077g) is then mixed with 50 ml acetic acid and 100 ml of distilled water with constant stirring of about 3 hours. The sample is then filtered by using Buckner funnel. The residue is then washed with distilled water for 10 times. The sample is then placed in the oven at 100˚C for 6 hours. For the thermal treatment of the sample it is placed in the muffle furnace at 150˚C for 5 hours. The synthesis routes are tabulated below
Table 1: Synthesis route of Hap from egg shells
|
Sample
|
Synthesis route
|
|
A
|
Egg shell + hexane
|
|
B
|
Egg shell + acetic acid (1000 oC , 2hrs)
|
|
C
|
Egg shell + hexane ( 1000 oC)
|
|
D
|
Egg shell + acetic acid + distil water (100oC, 6hrs)
|
Proto XRD at Centre of Excellence for Green Energy and Efficient Technology (CoE-GEET) CUJ was used for the XRD studies where monochromatic Cu-Kα radiation having a wavelength of 1.54Å was used. The XRD plots of the samples prepared by four different routes given in Fig 1 were indexed from published reports.8 The peaks in sample A, C and D were of Calcium Carbonate (CaCO3) in calcite phase.9 Evidence of HAP was obtained in sample B. This section is stored in ref 10
Figure 1: XRD of HAp synthesized by various routes
Zinc Oxide (ZnO) has been a promising material showing a lot of application. Various methods and processes have been proposed for its synthesis in nanoparticle form.11 We have used three different preparation techniques viz Co-precipitation method, Sol-gel and green synthesis and compared the XRD results.12–27
Preparation of ZnO nanoparticles by Co- precipitation method
The procedure as per ref15 was followed with slight alterations. The solution was prepared with de-ionized distilled water. First of all Zinc nitrate hexahydrate (Zn(NO3)2..6H2O) of 0.1M (2.97gm) and 0.8M (3.2gm) of Sodium hydroxide (NaOH) were separately dissolved in each 100ml of distilled in 100ml of beaker. And treated bymagnetic stirrer. NaOH solution was added dropwise to that solutionwhich produceda white precipitate. The solution withwhite precipitate was stirred at room temperature for 48 hrs. The solution became dried inside beaker and finally ZnO nanoparticle powder were obtained (Fig 2).
Figure 2: Prepared ZnO at Lab by co-precipitation method
Preparation of ZnO nanoparticles by Sol- Gel method
The zinc oxide nanoparticles were readily synthesized through sol-gel method using zinc acetate as a precursor.The procedure as per ref 19 was followed with alterations in the amount of materials used. In a typical procedure, an aqueous solution made of1.26g of zinc acetate dehydrate was heated followed by slow addition of absolute alcohol and 600 µL of H2O2 (Fig 3).This solution was dried to obtain white nano zinc oxide.19
The crystalline structure, morphology of synthesized ZnO nanoparticles were observed using powder X-ray diffraction (XRD).
Figure 3: (a) Shows ZnO solution during preparation, (b) Shows ZnO dried at 80 oC.
Preparation of ZnO nanoparticles by Green synthesis method
Sample HIBISCUS (H1)
10ml of Hawaiian hibiscus leaves extract was taken and boiled to 60-80 degree Celsius with a stirrer-heater. About 1.2g of Zinc Nitrate was added slowly to the resolution as the temperatures reached 60 oC. The procedure followed was as per ref.13
Sample HIBISCUS (H2)
10 ml of Hawaiian Hibiscus leaves extract was again taken and the same procedure was repeated.13 But this time dilute 1.2 g of zinc nitrate in 10 ml of distilled water was taken and dissolved using magnetic stirrer.
This mixture was then boiled till a deep yellow colored glue was formed which was heat treated at 400 oC for 2 hours. A light yellow colored powder was obtained as reported earlier13 (Fig 4(b)).
Sample TULSI (T1)
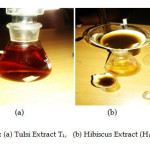
The procedure followed was as per ref. 14 The leaves of Ocimum Tenuiflorum (Tulsi)were washed and dried in sunlight. And their solution in distilled water was boiled the colour turns in reddish (Fig 4 (a)). The solution was then cooled at room temperature. About 1.2 g Zinc nitrate was further used to form Zinc oxide nanoparticles obtained as light yellow coloured powder as per procedures reported in ref 14
Figure 4: (a) Tulsi Extract T1, (b) Hibiscus Extract (H1,H2)
XRD analysis of ZnO via Co- Precipitation route
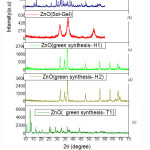
Proto XRD at Centre of Excellence for Green Energy and Efficient Technology (CoE-GEET) CUJ (Fig 1) with monochromatic Cu-Kα radiation having a wavelength of 1.54Å was used for the XRD studies.Fig 5a) shows the XRD pattern of ZnO synthesized by co-precipitation methodwhereintense and sharp peaks determined its purity and crystalline nature
XRD of ZnO via Green- Synthesis method
For sample H1 & H2, the XRD peaks appeared for 2θ value for H1 and H2 as shown in Fig 5 (c, d) are given in Table 2.For sample T1, the diffraction peaks were again intense and sharp in nature indicating formation of multiple phases.
Table 2: XRD peaks for sample H1 and H2
|
Sample
|
2θ
|
|
H1
|
31.88°,
|
34.46°,
|
36.41°
|
47.61°,
|
56.76°,
|
62.89
|
–
|
68.05
|
69.21
|
|
H2
|
31.98
|
34.61
|
36.48
|
47.81
|
56.81
|
63.06
|
66.58
|
68.14
|
69.33
|
Figure 5: XRD (multi- curve) of ZnO prepared by different synthesis methods
XRD of ZnO via Sol-Gel route
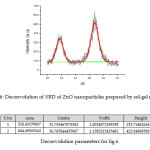
Fig 5 (b) shows a typical XRD pattern of ZnO nanoparticles prepared by Sol-gel route. A deconvoluted peak of the XRD of ZnO-NPs prepared as per sol gel is given in Fig 6 with parameters given in Table 2. Bragg reflections with 2θ values of 31.73°and 36.76° are observed corresponding to (100) plane for peak at 31.73°, (101) plane for intense peak at 36.76° were observed.. Average crystallite size of zinc oxide nanoparticles was determined as 79.25 nm from width of the dominate peaks (100) and (101) reflections according to Debye Scherrer equation. L = 0.9 λ /β Cos θ, where λ is the wavelength of X rays used (1.54060 Å), β is the full width at half maximum (FWHM) = 0.19184 and θ is the angle of diffraction.The broadening of the peaks in the XRD pattern can be attributed to the small particle size of the synthesized ZnO.
Figure 6: Deconvolution of XRD of ZnO nanoparticles prepared by sol-gel method.
Reference
- Reichert J., Binner J. G. P. Journal of Materials Science. 1996;31(5):1231-1241.
CrossRef
- Salah A. T., Mohammad M. A., Hassan A. M., El-Anadouli E. B. Development of nano-hydroxyapatite. chitosan composite for cadmium ions removal in wastewater treatment Journal of the Taiwan Institute of Chemical Engineers. 2014;45( 4):1571–1577.
- Ozawa M., Hattori M.,Satake K. Waste management and application of fish bone hydroxyapatite for waste water treatment Proceedings of International Symposium on EcoTopia Science 2007; 7 ISETS0 2007.
- Shu-Dong J., Qi-Zhi Y., Gen-Tao Z and Sheng-Quan F. J. Phys. Chem. C. 2012;116(7):4484–4492.
CrossRef
- Intapong S. Anirut Raksudjarit Treatment of Agricultural Wastewater using Porous C eramics Composite of Hydroxyapatite and Silica. Advanced Materials Research. 2013;622-623:915-918. Trans Tech Publications, Switzerland.
- Li Y., Zhou H., Zhu G., Shao C., Pan H., Xu X., Tang R. High efficient multifunctional Ag3PO4 loaded hydroxyapatite nanowires for water treatment. J Hazard Mater. 2015;299:379-87. doi: 10.1016/j.jhazmat. 2015.06.032. Epub 2015 Jun 23.
CrossRef
- Rioera E. M., Araiza M., Brostow W., Castano M. V., Estrada J. R. D., Hernandez R., Rodrıguez R. J. Synthesis of hydroxyapatite from eggshells. Materials Letters. 1999;41:128–134.
- Poinern E. G. J., Fawcett D. American Journal of Materials Science. 2013;3(5):130-135.
- Rahman A. M., Halfar J., Shinjo R. X-Ray Diffraction Is a Promising Tool to Characterize Coral Skeletons. Advances in Materials Physics and Chemistry. 2013;3:1.
- Sharma S.,Bhattacharyya A. S. Hydroxyapatite for Water Treatment. eprint Condensed Matter> vi Xra. 2016;1604:17.
- Mohammad V., Umar A. Yoon-Bong H. ZnO Nanoparticles: Growth, Properties and Applications In book: Metal Oxide Nanostructures and Their Applications, Chapter: 4, Publisher: American Scientific Publishers. 1-36.
- Kundu K. T., Karak N., Barik P., Saha S. Optical Properties of Zno Nanoparticles Prepared by Chemical Method Using Poly (VinylAlcohol ) (PVA) as Capping Agent. International Journal of Soft Computing and Engineering (IJSCE) ISSN. NCRAMT. 2011;(1):2231-2307.
- Winifred O.,Phyllis S., Lucinda F. Green Fusion of Zinc Oxide Nano particles with Hawaiian Hibiscus ,SSRG International Journal of Thermal Engineering (SSRG-IJTE). 2015;1(4).
- Raut S., Thorat P. V.,Thakre R. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Ocimum Tenuiflorum Leaves. International Journal of Science and Research (IJSR). 2013;6:14. ISSN (Online): 2319-7064,Index Copernicus Value. Impact Factor. 2013;4:438.
- Devi G. P., Velu S. A. Synthesis by co-precipitation method, structural and optical properties of ZnO and nickel doped ZnOnanoparticles. Journal of Advanced Applied Scientific Research – ISSN: 2454-3225. Geetha Deviet. al JOAASR. 2015;1-2:130-137.
- Devi S. Ȧ. R and Gayathri Ȧ. R. Green Synthesis of Zinc Oxide Nanoparticles by using Hibiscus rosa-sinensis, International Journal of Current Engineering and Technology E-ISSN 2277 – 4106, P-ISSN 2347 – 5161 ©2014 INPRESSCO®.
- Shah K. R., Boruah F., Parween N. Synthesis and Characterization of ZnO Nanoparticles using Leaf Extract ofCamellia sinesis and Evaluation of their Antimicrobial Efficacy, ISSN: 2319-7706. 2015;4(8):444-450. http://www.ijcmas.com
- Bhowmik S., Chowdhury S. D., Kabir M. H and Ali7 M. A. Chemical composition of some medicinal plant products ofindigenous origin. Department of Poultry Science, Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh.
- Alwan M. R., Kadhim A. Q., Sahan M. K., Ali A. R., Mahdi J. R., Kassim A. N., Jassim N. A. Synthesis of Zinc Oxide Nanoparticles via Sol – GelRoute and Their Characterization. Nanoscience and Nanotechnology. 2015.
- Kumar S. S., Venkateswarlu P., Rao R.V and Rao N. G. Synthesis, characterization and optical properties of zinc oxide nanoparticles, Kumar et al. International Nano Letters. 2013.
CrossRef
- Prakoso P. S., Saleh R. Synthesis and SpectroscopicCharacterization ofUndoped Nanocrytalline ZnO Particles Preparedby Co-Precipitation. Materials Sciences and Applications. 2012;3:530-537.
CrossRef
- Baruah S & Dutta J. Hydrothermal growth of ZnO nano structures. Science and Technology of Advanced Materials. 2009;10:1:013001.
- Vanaja A., Rao S. K. Effect of Co Doping on Structural and Optical Properties of Zinc Oxide NanoparticlesSynthesized by Sol-Gel Method. Advances in Nanoparticles. 2016;5:83-89.
CrossRef
- Singha C. R.,Author V., Author Vitae S. O., Singh P. M., Author Vitae b., Singh P., Author Vitae C. Synthesis of zinc oxide nanorods and nanoparticles by chemical route and their comparative study as ethanol sensors. Sensors and Actuators B: Chemical. 2008;135(1):352–357.
CrossRef
- Singh P. R. P., Hudiara I. S., Panday S., Kumar P., Rana S. B. Influence of Co-doping on the Structural, Optical and Magnetic Properties of ZnO Nanoparticles . Int. J. Nanoelectronics and Materials. 2016;9:1-8.
- Muthukumaran S., Gopalakrishnan R. Structural, FTIR and photoluminescence studies of Cu doped ZnO nanopowders by co-precipitation method. Optical Materials. 2012;34:1946–1953.
CrossRef
- Castillo H. J., Rodriguez J. F., López-Malo V. A ., Sanchez-Mora E., Quiroz A. M and Bandala R. E. Synthesis, Structural Characterization and Photocatalytic Activityof Iron-Doped Titanium Dioxide Nanopowders. Journal of Technology Innovations in Renewable Energy. 2015;4:000-000.
Views: 2,377
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal