Physicochemical Analysis of Ground Water Samples of South Coastal Areas of Kanniyakumari in the Post-Tsunami Scenario
Krishna Prakash Arunachalam1, Monikandon Sukumaran2*, Mohammad Tanveer3 and Kesavan Devarayan4*
1Department of Civil Engineering, University College of Engineering, Nagercoil, Kanniyakumari 629 004, India
2Department of Basic Engineering, College of Fisheries Engineering, Tamil Nadu Fisheries University, Nagapattinam 611001, India
3Department of Aquacultural Engineering, College of Fisheries Engineering, Tamil Nadu Fisheries University, Nagapattinam 611001, India
4Department of Basic Sciences, College of Fisheries Engineering, Tamil Nadu Fisheries University, Nagapattinam 611001, India
Corresponding Author Email: monicivil.engineer@gmail.com
DOI : http://dx.doi.org/10.13005/msri/130209
Article Publishing History
Article Received on : 8 Aug 2016
Article Accepted on : 14 Aug 2016
Article Published : 06 Sep 2016
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
In this study, the effect of ‘natural/self cleaning’ of ground water by precipitation after a decade of tsunami is evaluated along the coast of Kanniyakumari district, Tamil Nadu, India. The samples were collected from five stations namely Colachel, Muttom, Periyakadu, Manakudy, and Kanniyakumari. The physicochemical parameters such as turbidity, total dissolved solids, electrical conductivity, pH, alkalinity, total hardness, calcium, magnesium, sodium, potassium, chloride, and fluoride were evaluated and compared with the database of the pre-tsunami sample collected in 2004. The results indicated that the ‘natural cleaning’ has improved the quality of the ground water over the years after tsunami.
KEYWORDS:
Ground Water Quality; Kanniyakumari; Natural Cleaning; Physicochemical Parameters; Tsunami
Copy the following to cite this article:
Arunachalam K. P, Sukumaran M, Tanveer M, Devarayan K. Physicochemical Analysis of Ground Water Samples of South Coastal Areas of Kanniyakumari in the Post-Tsunami Scenario. Mat.Sci.Res.India;13(2)
|
Copy the following to cite this URL:
Arunachalam K. P, Sukumaran M, Tanveer M, Devarayan K. Physicochemical Analysis of Ground Water Samples of South Coastal Areas of Kanniyakumari in the Post-Tsunami Scenario. Mat.Sci.Res.India;13(2). Available from: http://www.materialsciencejournal.org/?p=4465
|
Introduction
Generally, groundwater is considered as an important drinking water source, especially in coastal regions due to limited availability of surface water. Kanniyakumari district is situated in the farthest south of Tamil Nadu, India. Even though abundant surface water is available in this district, the major population prefers to use ground water. For instance, almost every home situated in this district uses ground water either from open wells or from bore wells. This is due to the availability of the ground water with acceptable drinking quality which is recharged by South-West and North-South monsoons.
In 2004, an earthquake occurred in the west coast of Sumatra Indonesia which triggered a series of devasting tsunami and affected quality of water and soil along the coast of most of Indian Ocean. Since then many researchers evaluated the effect of tsunami on water quality till today. The results indicated that the physicochemical parameters such as total dissolved solids (TDS), electrical conductivity (EC), sulphate, nitrate, phosphate, chloride, fluoride, alkalinity, and total hardness were well above the permissible limit suggested by WHO. Hence the ground water along the coast of India1-6 Indonesia,7 Thailand,8 and Sri Lanka9 were found to be severely contaminated.
Similarly, the ground water of Kanniyakumari district was also reported as not preferable for irrigation, domestic, and industrial purposes.10,11 However, the quality of water can change with time significantly by natural climatic forces. Unlike other districts of the province Tamil Nadu of India, Kanniyakumari receives more rainfall (Table 1,12). In this view, the current status of the ground water quality and the effect of ‘natural/self cleaning’ response by precipitation over a decade are evaluated.
Materials and Methods
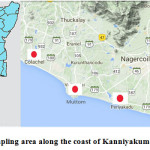
Kanniyakumari district is located in the southern part of Tamil Nadu province at 8.078°N 77.541°E. It is one of the areas that was affected by tsunami in 2004. Enormous amounts of sea water entered into the habitable land (0.5 – 2.0 km) along the south coast of the district. The details of sampling points are indicated in the Figure 1 and Table 2. For the purpose of comparison, the pre-tsunami data for water quality parameters for the year 2004 were obtained from Tamil Nadu Water Supply and Drainage Board, Nagercoil. In the present study, the water samples were collected for six months from December 2015 to May 2016. The samples were analyzed for various physicochemical parameters such as turbidity, TDS, EC, pH, alkalinity, total hardness, calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chloride (Cl), and fluoride (F) using APHA standards.13
Results and Discussion
Total Dissolved Solids
The values of total dissolved solids (TDS) are given in Table 3 and Figure 2a. The TDS of the sample collected in 2016 in 4 out of the 5 stations exhibited low values. Whereas the water samples collected from station 1 showed significantly higher values of TDS than the corresponding values in 2004. It is obvious that the ‘self cleaning’ effect of rain fall could have decreased the TDS content in station 2-5. However, in station 1, the TDS levels are still high. This may be due to the inundation of massive quantities of sea water followed by post-tsunami infiltration of the stagnated in flowed-sea water into the ground.
Electrical Conductivity
The electrical conductivity for stations 3, 4, and 5 were relatively lower than their corresponding values during 2004. Meanwhile, a significant increase in the EC values were observed for stations 1 and 2. Aforementioned, similar to station1, there was an intrusion of large quantities of sea water during 2004 tsunami into station 2 also. This may have caused increase in the EC of water collected from station 2.
pH and Alkalinity
On one hand, Figure 2c indicated that pH of the water samples pre-tsunami and 2016 does not significantly change. The pH of all the samples of 2016 was weakly alkaline ranging from 6.9 to 7.74. On the other hand, there is not much significant change in the values of alkalinity (Table 2 and Figure 2d) for samples collected in 2004 and 2016. The results of pH and alkalinity are coherent in nature.
Minerals
The quantities of Ca, Mg, Na, K, Cl, and F for the samples collected in 2004 and 2016 are given in Table 2 and Figure 2e&f. Apart from stations 1 and 2, in all other 3 stations, the amount of minerals such as Ca, Mg, and K were slightly higher in samples of 2016 than 2004. It is interesting to note that the amounts of Cl found in the samples of station 1 and 2 in 2016 is greatly higher than the values found in 2004. It may be attributed to the application of large quantities of chlorine for disinfection in station 1 during post-tsunami activities which might have formed compounds containing chlorides.
Figure 1: Sampling area along the coast of Kanniyakumari district.
Figure 2: Physicochemical parameters of water samples analyzed pre-tsunami (2004) and 2016. (a) TDS, (b) EC, (c) pH, (d) alkalinity, (e) quantity of minerals in 2004 samples, (f) quantity of minerals in 2016 samples, and (g) TH.
Total Hardness
The total hardness (TH) values for the water samples follow similar trend to the TDS (Figure 2g). The TH values are relatively higher for stations 1 and 2. Whereas, the TH values for all the other 3 stations are more or less close to each other.
Table 1: Total rainfall time series (2004 – 2015) data in Kanniyakumari district [12].
|
S.no.
|
Year
|
Total rainfall (mm)
|
|
1
|
2004
|
1463.8
|
|
2
|
2005
|
1791.3
|
|
3
|
2006
|
1654.7
|
|
4
|
2007
|
1558.5
|
|
5
|
2008
|
1674.7
|
|
6
|
2009
|
1301.0
|
|
7
|
2010
|
2105.6
|
|
8
|
2011
|
1186.6
|
|
9
|
2012
|
903.4
|
|
10
|
2013
|
1132.2
|
|
11
|
2014
|
1541.1
|
|
12
|
2015
|
2066.9
|
Table 2: Details of sampling stations
|
Station no.
|
Sampling location
|
Source
|
|
1
|
Colachel
|
Open well
|
|
2
|
Muttom
|
Open well
|
|
3
|
Periyakadu
|
Open well
|
|
4
|
Manakudy
|
Open well
|
|
5
|
Kanniyakumari
|
Open well
|
Table 3: Physicochemical parameters of water collected at 5 different stations during 2016 in coastal area of Kanniyakumari district.
|
Parameters
|
Station 1
|
Station 2
|
Station 3
|
Station 4
|
Station 5
|
|
Turbidity (NTU)
|
3
|
3
|
2
|
1
|
2
|
|
Total dissolved solids, TDS, mg/L
|
1975
|
1371
|
777
|
770
|
510
|
|
Electrical conductivity (mg/L)
|
2993
|
2077
|
1177
|
1167
|
773
|
|
pH
|
7.74
|
6.9
|
7.24
|
7.21
|
7.2
|
|
Alkalinity (mg/L)
|
128
|
372
|
484
|
284
|
248
|
|
Total hardness (mg/L)
|
980
|
640
|
360
|
320
|
172
|
|
Ca (mg/L)
|
104
|
72
|
40
|
48
|
34
|
|
Mg (mg/L)
|
173
|
110
|
62
|
48
|
21
|
|
Na (mg/L)
|
230
|
172
|
78
|
92
|
83
|
|
K (mg/L)
|
33
|
32
|
22
|
23
|
16
|
|
Cl (mg/L)
|
890
|
460
|
90
|
170
|
86
|
|
F (mg/L)
|
0.2
|
0.4
|
0
|
0.2
|
0.2
|
Table 4: Physicochemical parameters of water collected in Kanniyakumari district during 2004 (before tsunami).
|
Parameters
|
Station 1
|
Station 2
|
Station 3
|
Station 4
|
Station 5
|
|
Turbidity (NTU)
|
3
|
2
|
1
|
2
|
3
|
|
Total dissolved solids, TDS, mg/L
|
322
|
845
|
608
|
820
|
403
|
|
Electrical conductivity (mg/L)
|
1330
|
652
|
1170
|
1700
|
900
|
|
pH
|
7
|
8.2
|
7.5
|
6.9
|
7.7
|
|
Alkalinity (mg/L)
|
228
|
342
|
420
|
264
|
512
|
|
Total hardness (mg/L)
|
417
|
314
|
730
|
165
|
252
|
|
Ca (mg/L)
|
83
|
180
|
39
|
43
|
167
|
|
Mg (mg/L)
|
24
|
33
|
80
|
41
|
32
|
|
Na (mg/L)
|
76
|
69
|
104
|
53
|
92
|
|
K (mg/L)
|
15
|
6.5
|
13
|
8
|
14.8
|
|
Cl (mg/L)
|
259
|
126
|
156
|
129
|
48
|
|
F (mg/L)
|
0.25
|
0.21
|
0.3
|
0.2
|
0.23
|
Conclusion
In this study, the ground water samples were collected from 5 different stations located along the coastal areas of Kanniyakumari district. As per APHA standards, the samples were analyzed for
physicochemical parameters such as turbidity, TDS, EC, pH, alkalinity, total hardness, calcium, magnesium, sodium, potassium, chloride, and fluoride. The results are compared with the quality of water pre-tsunami in 2004. The results indicated for decrease or more or less similar values for almost all of the physicochemical parameters. This is attributed to the ‘natural/self cleaning’ effect of the rain fall for the past 12 years, which could have leached out the salts and minerals to restore the quality of the water. It is proposed that the contamination in the ground water may get better if there is no intervention by anthropogenic activities and natural calamities.
Acknowledgement
The authors are thankful to College of Fisheries Engineering, Tamil Nadu Fisheries University for motivation towards research.
References
- Chandrasekharan H, Sarangi A, Nagarajan M, Singh V P, Rao D U M, Stalin P, Natarajan K, Chandrasekaran B, Anbazhagan S, J Environ Manage. 2008;89:63-72.
CrossRef
- Srinivasalu S, Thangadurai N, Jonathan M P, Armstrong-Altrin J S, Ayyamperumal T, Ram-Mohan V, Environ Geol. 2008;53:1711-1721.
CrossRef
- Kume T, Umetsu C, Palanisami K, J Environ Manage. 2009;90:3147-54.
CrossRef
- Mondal N C, Singh V P, Singh S, Singh V S, Environ Monit Assess. 2011;175:531-550.
CrossRef
- Sajil Kumar P J, Elango L, James E J, Arab J Geosci. 2014;7:2641-2653.
CrossRef
- Singaraja, C, Chidambaram S, Anandhan P, Prasanna M V, Thivya C, Thilagavathi R, Sarathidasan J, Arab J Geosci. 2014;7:939-950.
CrossRef
- Rajendran A, Mansiya C, Ecotoxicol Environ Saf, 2015;121:218-222.
CrossRef
- Gupta S K, Suantio A, Gray A, Widyastuti E, Jain N, Rolos R, Hoekstra R M, Quick R, Am J Trop Med Hyg. 200;76:1158-62.
- Vaccari M, Collivignarelli C, Tharnpoophasiam P, Vitali F, Environ Monit Assess. 2011;161:123-133.
CrossRef
- Rossetto T, Peiris N, Pomonis A, Wilkinson S M, Del Re D, Koo R, Gallocher S, Nat Hazards. 2007;42:105-124.
CrossRef
- Venkatesh P, Murugesan S, Dhamotharan R, Biosci Biotech Res Comm. 2010;3:185-190.
- Ramesh K, Nithya K, Vennila S, Int J ChemTech Res. 2014;6:4585-4594.
- http://www.kanyakumari.tn.nic.in/climate.html
- American Public Health Association. “APHA Standard methods for the examination of water and wastewater.” American Water Works Association and Water Environment Federation. 1992.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal