Cocrystals: A Review of Recent Trends in Pharmaceutical and Material Science Applications
Introduction
The advent of crystal engineering has transformed the pace of research in molecular solids. Attaining the desired properties in molecular solids by controlled manipulation of intermolecular interactions holds the key to success in this area of research.1-2 For that matter, strength and directionality of weak intermolecular interactions are important features to be taken into account. Crystal engineering, through supramolecular synthons, allows us to design cocrystals with desired physicochemical properties.3-5 Cocrystallization6-8 and polymorphism9-11 are two important applications of crystal engineering. A cocrystal entails two or more neutral molecules to be held together by weak intermolecular interactions in a definite ratio. Since intermolecular interactions guide properties of molecular solids, cocrystal formation is of great interest to crystal engineers. Primary non-covalent interactions such as strong hydrogen bonds decide the framework of multicomponent crystal and secondary, weaker interactions such as halogen bonds, van der Waals forces act as auxiliary scaffolds to stabilize the framework12 Initially, cocrystals have been exploited in pharmaceutical research to alter the physicochemical properties of a drug such as bioavailability, solubility, stability, melting point and shelf life13-15 With this growing success in pharmaceuticals,16-17 cocrystallization as a tool to alter the properties of solids is spreading into research in material science. It is now being used in the design of organic functional materials and energetic materials. Charge transfer cocrystals18-20 have found a place in the design of optoelectronic devices such as Field effect transistors (FETs), diodes. Many of the energetic materials are highly sensitive and possess low power and undesirable detonation properties. Cocrystal formation of an energetic substance with suitable cocrystal former can greatly alter its sensitivity and density (and hence the power).21-26 More recently, researchers have opened up a new class of cocrystals called ternary cocrystals, which involves binding of three neutral molecular entities through weak interactions which are interesting as well as challenging. Over the years, cocrystallization has been used as a tool to vary the properties of a solid and hence this area has generated a lot of interest and attention. With the help of crystal structure prediction (CSP), it is now possible to know the crystal structure and motifs involved in the framework of cocrystals.27 A crystal engineer can have an idea if the experiments involving high-cost drug molecules and other compounds will yield fruitful results. Here we present a brief review of pharmaceutical and material science applications of cocrystals.
Pharmaceutical cocrystals
A pharmaceutical cocrystals a homogeneous crystalline entity which contains an active pharmaceutical ingredient (API) and a cocrystal former (GRAS molecule) in a stoichiometric ratio, in the same crystal lattice stabilized by non-covalent interactions such as hydrogen bonds, π−π interactions, ionic interactions, and van der Waals forces.28-30 The majority of the drug molecules in the market are sold out in their salt forms, and some molecules, though potential drug candidates, cannot form salts due to the absence of ionizable sites in the molecule. Cocrystallization comes in handy in such cases, which allows us to use them as neutral molecules along with coformers without loss of biological activity.
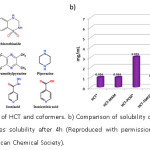
The majority of the drugs are ineffective in their bioactivity due to low solubility and permeability, which limits their clinical applications. Over the years, cocrystallization is widely used in the field of pharmaceuticals for improving the physicochemical properties of the drug molecules such as solubility, bioavailability, permeability, stability, melting point and shelf life.31-32 Because of the simple and profitable methods involved in designing a cocrystal, and performance, it is now a most viable method for improving the physicochemical properties of drugs. It is to be noted that, there is no particular method to design cocrystals of all the drugs. The strategy which works for one may not work for the other; each drug has to be dealt separately keeping the property to be varied in mind. The solubility of a drug depends on melting point, dielectric constant, temperature, and pH. Recently, new cocrystals of Hydrochlorothiazide (HCT) with piperazine (PPZ), tetramethylpyrazine (TMPZ), picolinamide (PCM), malonamide (MAM), isonicotinic acid (INIC), and with a tuberculosis drug isoniazid (INZ) were reported,33 among them HCT-PPZ showed seven-fold increase in solubility (Figure 1). Permeability is another important property of an API which depends on absorption of the drug on the cell membrane. Both solubility and permeability were enhanced in the case of HCT-PPZ which is essential for bioavailability of the drug. It can be clearly seen that cocrystals always need not have a higher solubility than the drug (HCT-MAM and HCT-TMPZ have solubilities lower than HCT).
Figure 1: a) Structure of HCT and coformers. b) Comparison of solubility of HCT and cocrystals. Asterisk mark denotes solubility after 4h (Reproduced with permission from reference 33. Copyright 2017 American Chemical Society).
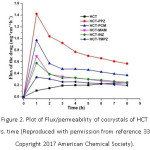
The diffusion behavior of the new solid forms of HCT was studied using a Franz diffusion cell, the plot of flux of the drug at the time indicate that HCT−PPZ and HCT−PCM cocrystals exhibit better diffusion behavior among all the cocrystals (Figure 2). It is suggested that coformers that cause higher solubility in cocrystals lead to higher diffusion.
Figure 2: Plot of Flux/permeability of cocrystals of HCT vs. time (Reproduced with permission from reference 33. Copyright 2017 American Chemical Society).
Our group has reported ten different cocrystals,one polymorph of a cocrystal and a salt of an organofluorine drug riluzole with various carboxylic acids and pyridine derivatives.12 The new crystalline phases are stabilized by primary and secondary intermolecular interactions, with a melting point range between 90-140˚C closely related to MP of original API (118°C). Cocrystals exhibited enhanced solubility and dissolution rate in comparison to riluzole.12 The structure of compounds used in the study and the plot of the cumulative amount of cocrystal dissolved against time is given in Figure 3a and 3b respectively.
Figure 3: a) Structure of the drug riluzole and coformers used in the study. b) The intrinsic dissolution rate Measurements of riluzole cocrystals and salt in pH 7 buffer medium (Reproduced with permission from reference 12. Copyright 2017 American Chemical Society)
Charge Transfer cocrystals
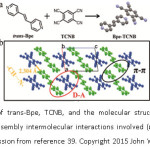
Single component organic materials used in designing organic semiconductors have attained limited success. Organic cocrystals involving ordered stacking of donors and acceptors offer unique physicochemical properties which are desirable for applications in optoelectronic devices (like Field Effect Transistors and optical diodes).34-35 This has led to growing interest in yet another nascent subclass of cocrystals, namely, charge transfer cocrystals which have greater implications for designing optoelectronic devices. The most important factor as far as charge transfer cocrystals are concerned is ionicity or degree of charge transfer between a donor (D) and an acceptor (A). Ionicity or degree of CT is directly related to conducting ability36-37 and lattice instability38 of cocrystals. As of now, there exists no particular method for confirming the degree of charge transfer, and its elusive nature requires further research in this area. Hu et al, have reported a novel, 2-D light emitting charge transfer (1:1) cocrystal of a 1,2-di(4-pyridyl)ethylene (Bpe) and 1,2,4,5-tetracyanobenzene (TCNB) molecules (Figure 4).39
Figure 4: a) Structure of trans-Bpe, TCNB, and the molecular structure Bpe-TCNB complex obtained. b) The self-assembly intermolecular interactions involved (π···π, D···A, and C-H···N) (Reproduced with permission from reference 39. Copyright 2015 John Wiley and Sons).
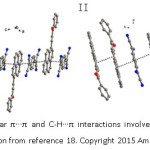
They have noted that “To the best of our knowledge, the CT nature of crystals of Donor- Acceptor complexes is difficult to verify accurately.” Both spectroscopic and theoretical methods were used to confirm the charge transfer nature in BPe-TCNB cocrystals. Xu et al18 have demonstrated the role of solvents in controlling the molar ratio and different charge transfer interaction modes by studying 1-phenyl-3-(pyren-1yl) prop-2-en-1-one (PPPO) as electron donor (D) and 7,7,8,8-tetracyanoquinodimethane (TCNQ) as electron acceptor (A) to form CT complexes (Figure 5).18
Figure 5: The intermolecular π···π and C-H···π interactions involved in CT complexes I and II (Reproduced with permission from reference 18. Copyright 2015 American Chemical Society).
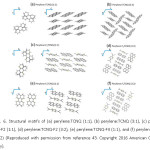
In recent years, the design of charge transfer cocrystals involving the use of conjugated aromatic hydrocarbons as electron donors and halogenated acceptors finds applications in electronics and photonics.40-42 Due to its excellent efficiency and stability of assembling cocrystals with electron donors, TCNQ is one of the most widely used acceptors in the field of CT complexes. Recently, E. D. Como and coworkers43 have synthesized CT cocrystals of perylene with TCNQ and its fluorine derivatives (Figure 6). Fluorine derivatives have strong acceptor abilities than TCNQ, leading to greater ionicity of CT interactions formed.
Figure 6: Structural motifs of (a) perylene:TCNQ (1:1), (b) perylene:TCNQ (3:1), (c) perylene: TCNQ-F2 (1:1), (d) perylene:TCNQ-F2 (3:2), (e) perylene:TCNQ-F4 (1:1), and (f) perylene:TCNQ-F4 (3:2) (Reproduced with permission from reference 43. Copyright 2016 American Chemical Society).
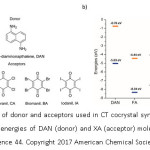
Very recently, Prof A. J. Matzger and co-workers have demonstrated the role of hydrogen bonding and halogen bonding on the packing and ionicity of CT- cocrystals.44 They have synthesized CT cocrystals using 1, 5-diaminonaphthalene (DAN) as the donor and halogen substituted benzoquinones (XA, X= F, Cl, Br, I) as acceptors (Figure 7a). A comparison of the HOMO and LUMO energies of DAN (donor) and XA (acceptor) molecules is given in Figure 7b.
Figure 7: a) Structures of donor and acceptors used in CT cocrystal synthesis. b) A Comparison of HOMO and LUMO energies of DAN (donor) and XA (acceptor) molecules (Reproduced with permission from reference 44. Copyright 2017 American Chemical Society).
In this work, the dependence of the degree of ionization of cocrystal on electronegativity of the halogen substituent was demonstrated experimentally by using Raman spectroscopy. For this study, anion radicals of CA and BA were synthesized and were subjected to Raman spectroscopy along with CA, BA, DAN−CA cocrystal and DAN−BA cocrystal. The corresponding carbonyl peak in each spectrum (CA = 1689.7 cm−1, CA•− = 1588.2 cm−1 and DAN−CA = 1663.3 cm−1; BA = 1681.3 cm−1, BA•− = 1582.3 cm−1 and DAN−BA = 1657.7 cm−1 (Figure 8a and 8b) was used to estimate ρ(ionicity) for both cocrystals. The more electronegative chlorine (3.0) substituted DAN−CA has an ionicity value of 0.27 e, and the less electronegative bromine (2.8) substituted DAN−BA has an ionicity value of 0.23 e, showing the direct relation between ionicity and electronegativity of halogen substituent.
Figure 8: (a) Raman spectra (Stacked) of the pure component CA, CA radical anion, and cocrystal (CA-DAN). The carbonyl peak used to estimate ionicity is marked by the red ring. (b) Raman spectra of the BA pure component, BA radical anion, and DAN−BA cocrystal. The carbonyl peak used to estimate ionicity is marked by the red ring (Reproduced with permission from reference 44. Copyright 2017 American Chemical Society).
Energetic cocrystals
Energetic materials, a class of materials which includes explosives, propellants and pyrotechnics suffer from undesirable properties such as poor stability, high sensitivity and low density( hence, low explosive power) which have limited their applications. Traditionally, the development and optimisation of properties of energetic materials was dependent on synthetic methods. In recent years, crystal engineering through cocrystallization has paved new ways to alter the physicochemical properties of energetic materials very efficiently. Cocrystallization offers a robust method for attaining desired sensitivity, stability, and high power energetic materials without requiring synthesis of new compounds.
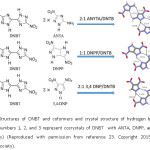
A particular class of energetic materials, azoles are of great interest from the point of crystal engineering as they possess hydrogen bond donors (primary and secondary amines in aromatic systems). Matzger and coworkers have reported a series of three cocrystals of 5,5′-Dinitro2H,2H′-3,3′-bi-1,2,4-triazole (DNBT), which is a high-density azole explosive, which has limited military applications.23 The structures of DNBT and three coformers: 5-amino-3-nitro-1H-1,2,4-triazole (ANTA), 1H,4H-3,6-dinitropyrazolo[4,3-c]pyrazole (DNPP), and 3,4-dinitropyrazole (3,4-DNP), and the hydrogen bond motifs utilized in cocrystal formation are given below in Figure 9.
Figure 9: Structures of DNBT and coformers and crystal structure of hydrogen bond motifs involved (numbers 1, 2, and 3 represent cocrystals of DNBT with ANTA, DNPP, and 3,4-DNP respectively) (Reproduced with permission from reference 23. Copyright 2015 American Chemical Society).
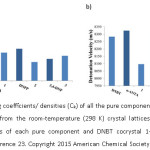
Further, packing coefficient (Ck) which is a measure of the density of an energetic material, is estimated (Figure 10a), and the detonation velocities of all the three cocrystals were determined using Cheetah 6.0 software at 298K (Figure 10b).23 All the cocrystals prepared have the power of pure components with decreased impact sensitivity which can be attributed to the hydrogen bonding interactions in the cocrystals.
Figure 10: a) Packing coefficients/ densities (Ck) of all the pure components and DNBT cocrystal 1−3 are calculated from the room-temperature (298 K) crystal lattices of each material. b) Detonation velocities of each pure component and DNBT cocrystal 1−3. (Reproduced with permission from reference 23. Copyright 2015 American Chemical Society)
Another area of concern regarding energetic materials is their sensitivity towards external stimuli which hampers storage and handling. Currently, there are two methods available for loading the energetic materials into munitions: a) melting and casting, b) hydraulic pressing.26 The former method is more advantageous than the latter in terms of reduction of void space and greater processing efficiency.
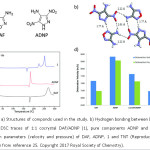
Matzger et al have designed an energetic cocrystal of 3, 4-diaminofurazan (DAF) and 4-amino-3,5-dinitropyrazole(ADNP) is melt castable, a property which is not possessed by pure components.25 The structure of compounds used and hydrogen bonding interactions is given (Figure 11a and 11b respectively). It is known that a good melt castable material must possess, a low melting point, large range between the melting point and the start of decomposition, and high loading density.26 As observed from DSC curves of 1, ADNP and DAF (Figure 11c), the cocrystal 1 has a melting point around 172˚C, which is lower than both ADNP and DAF. Further, there is a large difference in melting point and starting of decomposition, which is absent in ADNP. Thus, it qualifies to be a melt castable energetic material. In addition, theoretical estimation of detonation parameters (velocity and pressure) using Cheetah 7.0 software indicates the high performance of ADNP/DAF cocrystal, which is intermediate in comparison to the pure components (Figure 11d).
Figure 11: a) Structures of componds used in the study. b) Hydrogen bonding between DAF and ADNP. c) DSC traces of 1:1 cocrystal DAF/ADNP (1), pure components ADNP and DAF. d) Detonation parameters (velocity and pressure) of DAF, ADNP, 1 and TNT (Reproduced with permission from reference 25. Copyright 2017 Royal Society of Chemistry).
There is limited literature available on cocrystallization of energetic materials as this is a new area yet to be ventured into. Cocrystallization as a tool to vary the properties of energetic materials has gained attention in recent years. We hope to see more reports in this area in the coming years.
Ternary cocrystals
So far, we discussed about cocrystals involving two components and their importance. A large volume of literature is available on binary cocrystals. Two component molecular crystals are comparatively easy to synthesize and are established in terms of design and assembly.45,46 Ternary cocrystals is a recent advancement in crystal engineering and are yet to be established, hence there is lot of room for optimization of strategies for designing ternary cocrystals. A ternary cocrystal is an assembly of three neutral solid compounds in a single crystal structure through hydrogen bonding and other weak interactions.46 On moving from binary to a ternary system, there are a number of ways in which three components can assemble themselves in a single crystal lattice. This makes the process of design extremely difficult and challenging. Added to this, availability of specific and universal interactions may lead to the formation of polymorphs47-50 of individual molecules, binary cocrystals, solvatomorphs, etc. This renders design of ternary cocrystals more challenging. Desiraju and coworkers have carefully noted: ‘‘Making a stoichiometric ternary cocrystal from three substances that are all solids under ambient conditions is far more difficult and demands a good knowledge of the interaction preferences’’.45 A proper balance between intermolecular interactions and crystallization is necessary to obtain a ternary cocrystal. Ternary cocrystals provide a viable alternative to vary the physicochemical properties where binary cocrystals fail to do so.
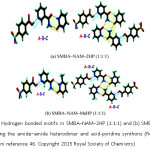
In 2015, A. Nangia and coworkers reported five novel ternary cocrystals of p-sulfonamide benzoic acid (SMBA) with pyridine amides and lactam coformers by exploiting supramolecular synthons of the carboxylic acid and sulfonamide functional groups.46 Two of which are shown in Figure 12.
Figure 12: (a) Hydrogen bonded motifs in SMBA–NAM–2HP (1:1:1) and (b) SMBA–NAM–MeHP (1:1:1) involving the amide–amide heterodimer and acid-pyridine synthons (Reproduced with permission from reference 46. Copyright 2015 Royal Society of Chemistry)
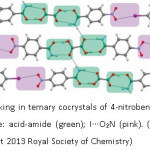
In their work on designing ternary cocrystals using hydrogen and halogen bonds, G.R. Desiraju and coworkers have carefully noted: “a balance of interactions and solubilities is needed to get a ternary cocrystal”.45 2:1:1 Ternary cocrystals of 4-nitrobenzamide: diacid: 1, 4-dihalogenated benzene was designed by graded selection of hydrogen and halogen bonds (Figure 13).
Figure 13: The crystal packing in ternary cocrystals of 4-nitrobenzene: oxalic acid: 1,4-diiodo-benzene (2:1:1). Color code: acid-amide (green); I···O2N (pink). (Reproduced with permission from reference 45. Copyright 2013 Royal Society of Chemistry)
Conclusions
In conclusion, cocrystallization has become a very useful tool for a crystal engineer to modulate the properties of solid state materials. Recent developments in this area such as CT cocrystals, energetic cocrystals, and ternary cocrystals are yet to be established. As of now, the results of applications of cocrystallization in pharmaceuticals are fruitful only at microscopic level. Strategies to produce cocrystals in larger scales are yet to be ventured into, in order to yield fruitful results of whatever has been achieved at microscopic level. Needless to say, in the coming year’s research in this area would be more focused on scaling up processes.
Acknowledgements
MGJ thanks NITK Surathkal. PKM thanks CSIR for the senior research fellowship. DC thanks, DST-SERB Scheme for funding. We are thankful to IISER Bhopal for research facilities and infrastructure.
References
- Desiraju G. R., Vittal J. J and Ramanan A. Crystal Engineering: A Textbook, World Scientific, Singapore. 2011.
CrossRef
- Desiraju G. R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam. 1989.
- Desiraju G. R. Angew. Chem.Int. Ed. 1995;34(21):2311−2327.
CrossRef
- MacNicol D. D., Toda F and Bishop R. Solid-State Supramolecular Chemistry: Crystal Engineering, Comprehensive Supramolecular Chemistry, ed., Pergamon, Oxford. 1996;6.
- Nangia A. J. Chem. Sci. 2010;122:295–310.
CrossRef
- McNamara D. P., Childs S. L., Giordano J., Iarriccio A., Cassidy J., Shet M. S., Mannion R., O’Donnell E., Park A. Pharm. Res. 2006;23:1888−1897.
CrossRef
- Sanphui P., Tothadi S., Ganguly S., Desiraju G. R. Mol. Pharmaceutics. 2013;10:4687−4697.
CrossRef
- Yan Y., Chen J. M., Lu T. B. Cryst. Eng. Comm. 2013;15:6457−6460.
CrossRef
- Mondal P. K & Chopra D. Mat. Sci. Res. India. 2014;11(1):43-50.
CrossRef
- Bernstein J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, Great Britain. 2002.
- Mondal P.K., Kiran M. S. R. N., Ramamurthy U and Chopra D. Chem. Eur. J. 2017;23:1023–1027.
CrossRef
- Mondal P. K., Rao V., Mittipalli S and Chopra D. Cryst. Growth Des. 2017;17:1938-1946.
CrossRef
- Duggirala N. K., Perry M. L., Almarsson O and Zaworotko M. J. Chem. Commun. 2016;52:640–655.
CrossRef
- Bolla G and Nangia A. Chem. Commun. 2016;52:8342.
CrossRef
- Saikia B., Bora P., Khatioda R and Sarma B. Cryst. Growth Des. 2015;15:5593−5603.
CrossRef
- Steed J. W. Trends Pharmacol. Sci. 2013;34:185–193.
CrossRef
- Goud N. R., Gangavaram S., Suresh K., Thapal S., Manjunatha S. G., Nambiar S and Nangia A. J. Pharm. Sci. 2012;101:664–680.
CrossRef
- Sun H., Wang M., Wei X., Zhang R., Wang S., Khan A., Usman R., Feng Q., Du M., Yu F., Zhang W and Xu C. Cryst. Growth Des. 2015;15:4032−4038.
CrossRef
- Zhu L. Y., Yi Y. P., Li Y., Kim E. G., Coropceanu V and Bredas J. L. J. Am. Chem. Soc. 2012;134:2340.
CrossRef
- Landenberger K. B and Matzger A. J. Cryst. Growth Des. 2010;10:5341.
CrossRef
- Landenberger K. B and Matzger A. J. Cryst. Growth Des. 2012;12:3603.
CrossRef
- Millar D. I. A., Maynard-Casely H. E., Allan D. R., Cumming A. S., Lennie A. R., Mackay A. J., Oswald I. D. H., Tang C. C and Pulham C. R. Cryst. Eng. Comm. 2012;14:3742.
CrossRef
- Bennion J. C., McBain A., Son S. F and Matzger A. J. Cryst. Growth Des. 2015;15:2545−2549.
CrossRef
- Klapotke T. M. Chemistry of High-Energy Materials, Walter de Gruyter, Berlin/Boston. 2012.
CrossRef
- Bennion J. C., Siddiqia Z. R and Matzger A. J. Chem. Commun. 2017. DOI: 10.1039/c7cc02636f
CrossRef
- Ravi P., Badgujar D. M., Gore G. M., Tewari S. P and Sikder A. K. Propellants, Explos., Pyrotech. 2011;36:393.
CrossRef
- Mondal P. K and Chopra D. Cryst. Eng. Comm. 2016;18:48.
CrossRef
- Almarsson O and Zaworotko M. J. Chem. Commun. 2004;1889–1896.
CrossRef
- Schultheiss N and Newman A. Cryst. Growth Des. 2009;9:2950–2967.
CrossRef
- Childs S. L., Kandi P and Lingireddy S. R. Mol. Pharmaceutics. 2013;10:3112−3127.
CrossRef
- Good D. J and Rodríguez-Hornedo N. Cryst. Growth Des. 2009;9:2252−2264.
CrossRef
- Aakeroy C. B., Forbes S and Desper J. J. Am. Chem. Soc. 2009;131:17048–17049.
CrossRef
- Gopi S. P., Banik M and Desiraju G. R. Cryst. Growth Des. 2017;17:308−316.
CrossRef
- Jiang H., Zhang K. K., Ye J., Wei F., Hu P., Guo J., Liang C., Chen X., Zhao Y., McNeil L. E., Hu W and Kloc C. Small. 2013;9:990–995.
CrossRef
- Yao W., Yan Y., Xue L., Zhang C., Li G., ZhengQ.,Zhao Y. S., Jiang H and Yao J. Angew.Chem. Int. Ed. 2013;52:8713–8717. Angew.Chem. 2013;125:8875–8879.
CrossRef
- Pope M. Electronic Processes in Organic Crystals and Polymers, 2nd ed., Oxford University Press, Oxford. 1999.
- Torrance J. B. Acc. Chem. Res. 1979;12:79–86.
CrossRef
- D’Avino G and Verstraete M. J. Phys. Rev. Lett. 2014;113:237602.
CrossRef
- Zhu W., Zheng R., Fu X., Fu H., Shi Q., Zhen Y., Dong H and Hu W. Angew. Chem. Int.Ed. 2015;54:6785–6789.
CrossRef
- Goetz K. P., Fonari A., Vermeulen D., Hu P., Jiang H., Diemer P. J., Ward J. W., Payne M. E., Day C. S., Kloc C., Coropceanu V., McNeil L. E., and Jurchescu O. D. Nat. Commun. 2014;5:5642.
CrossRef
- Park S. K., Varghese S., Kim J. H., Yoon S. J., Kwon O. K., An B. K., Gierschner J and Park S. Y. J. Am. Chem. Soc. 2013;135:4757.
CrossRef
- Sikdar N., Jayaramulu K., Kiran V., Rao K. V., Sampath S., George S. J and Maji T. K. Chem. Eur. J. 2015;21:11701.
CrossRef
- Salzillo T., Masino M., Kociok-Köhn G., Nuzzo D. D., Venuti E., Valle R. G. D., Vanossi D., Fontanesi C., Girlando A., Brillante A and Como E. D. Cryst. Growth Des. 2016;16:3028−3036.
- Goud N. R and Matzger A. J. Cryst. Growth Des. 2017;17:328−33.
CrossRef
- Tothadi S and Desiraju G. R. Chem. Commun. 2013;49:7791—7793.
CrossRef
- Bolla G and Nangia A. Chem. Commun. 2015;51:15578.
CrossRef
- Dey D and Chopra D. Cryst. Eng. Comm. 2015;17:5288.
CrossRef
- Sun C and Grant D. J. Pharm. Res. 2001;18:274.
CrossRef
- Davey R. J. Chem. Commun. 2003;1463.
CrossRef
- Swapna B., Suresh K and Nangia A. Chem. Commun. 2016;52:4037.
CrossRef
Views: 4,050
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal