Synthesis, Structural Characterization and Study of Biological Activity of Hydrazine Derivatives
Poonam Goklani*  and Anil Gupta
and Anil Gupta
P.G. Department of Chemistry, Govt. Dungar College, Bikaner-334001, India
Corresponding Author Email: poomam26wadhwani@gmail.com
DOI : http://dx.doi.org/10.13005/msri/140217
Article Publishing History
Article Received on : 27 Jul 2017
Article Accepted on : 23 Aug 2017
Article Published : 29 Aug 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Hydrazide-hydrazone derivatives play vital role in development of various pharmacological activities such as anti-tubercular, antiproliferative and antitumor activities. Some novel biologically active Hydrazide derivatives substituted with heterocyclic moiety have been synthesized. All the synthesized compounds structures were confirmed by IR, NMR and Mass spectra. Synthesized compounds were subjected to antibacterial screening in vitro and biological activity in vivo.
KEYWORDS:
Hydrazide derivatives; Pharmacological activity; Heterocyclic-moiety; spectral data
Copy the following to cite this article:
Goklani P, Gupta A. Synthesis, Structural Characterization and Study of Biological Activity of Hydrazine Derivatives. Mat.Sci.Res.India;14(2)
|
Copy the following to cite this URL:
Goklani P, Gupta A. Synthesis, Structural Characterization and Study of Biological Activity of Hydrazine Derivatives. Mat.Sci.Res.India;14(2). Available from: http://www.materialsciencejournal.org/?p=5816
|
Introduction
Hydrazine derivatives are very useful compounds. These are used in pharmaceutical, agrochemicals, polymer and dye industy.1 Hydrazines derivatives very tremendously not only as to modification in structure but also as regard to their therapeutic indication.2-4 Many of hydrazines having a simple structure are bacteriostatically active compounds which may be used as tuberculostatics, others are monoamine oxidase inhibitors, these are used as antidepressive.5 Some group of hydrazides has a clinically interesting saluretic activity. Many hydrazines with general formula R1R2NNR3R4 (R- H, alkyl, acyl or aryl substituent) shows incredible biological activity and are used in pharmaceutical. Some examples can refer to tuberculostatic activity of hydrazines.6,7
Hydrazines derivatives shows antimicrobial activits.8,9 N-N’-(carbazol-1-yl-1′,2′,3′,4′-tetrahydrocarbazol-1′-yl)hydrazine showed antibacterial activity against Escherichia coli, Staphylococeus aureus, Pseudomonas aerugimosa and Bacillus subtilis. These N-N’-carbazolyl hydrazine derivatives has been found to show antifungal activity against Aspergillus niger, Candida albicans.10 Isoquinolin-1-yl-2-(cycloalk-2-enylidene)hydrazines were prepared by the reaction of
1-hydrazino-3-isoquinoline with appropriate 1,3-diketones. These compounds also show good antibacterial and antifungal activity. Compound with electron rich groups exhibit antibacterial activity against bacterial species B. cerus.11
Some hydrazine derivatives are effective analgesics and are used as cyclooxygenase-2 (COX-2) inhibitors, what is essential for development of non-steroidal anti-inflammatory drugs.12 Substituted diphenylhydrazides are found to be effective pharmacophore for selective cyclooxygenase-2 (COX-2) inhibition, which is necessary in the design of novel anti-inflammattory drugs.12
N’-(2-phenoxybenzylidene)-2–phenoxybenzohydrazide, N’-(4-(methylthiobenzylidene)-2-phenoxybenzohydrazide, 2-(2-phenoxybenzylidene)-1-(2-nitrophenyl)hydrazine and 2-(2-phenoxybenzylidene)-1-(2,4-dinitrophenyl)hydrazine these compounds showed higher inhibitory effect against inflammation then mefenic acid (analgesic). It has been observed that attachment of phenoxy group in both 2 and 3 position of the phenyl ring can results in better analgesic activity.13
Acyl hydrazine derivatives exhibited excellent chemotherapeutic activity.14-17
Materials and Methods
All the chemicals and solvents were of reagent grade. Melting points were determined on a capillary melting apparatus and were uncorrected. H-NMR spectra were recorded on Bruker AMX-500 in CDCl3 with TMS as internal standard. IR spectra were recorded in KBr on a Perkin Elmer System 2000 FT-IR Spectrophotometer. For EI-mass spectra we use a VG Biotech Quattro 5022 spectrometer.
Some substituted hydrazine derivatives were synthesized by the diazotization method.18-20
Materials- Various amines, hydrochloric acid, sodium nitrite, sodium carbonate, sodium sulphite, stannous chloride, benzylhydrazine dihydrochloride , n-heptan and Triethylamine
Synthesis of Compounds
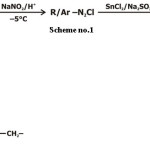
Compounds 1 and 2- These were synthesized by the following method.
Scheme 1
Some others have been synthesized by different methods.
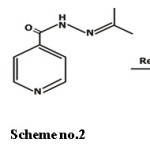
Compound 3– This compound is synthesized by the reaction of aryl hydrazide with 2-propanol or acetone and then followed by reduction.
Scheme 2
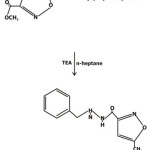
Compound 4– In this procedure methyl 5-methylisoxazole-3-carboxylate reacts with benzylhydrazine dihydrochloride and n-heptan. Triethylamine was added to the suspension slowly with a dropping funnel. At the end of the addition the temperature was adjusted to about 350C for 5-6 hours. Then cooled, filtered, washed with water and n-heptane and product obtained as white solid.
Scheme 3
Characterization
Chemical and spectral data of the compounds are shown in the following table (table: 1-3)
Table 1: Physiochemical Characterization Data For Compounds
|
Compound
|
Molecular Mass
g/mole
|
Elemental Analysis
|
Melting point 0C
|
Molecular Formula
|
|
|
|
C%
|
H%
|
N%
|
O%
|
|
|
|
1.
|
137
|
52.55
|
5.10
|
30.65
|
11.67
|
170-173
|
C6H7N3O
|
|
2.
|
122
|
68.85
|
8.19
|
22.95
|
–
|
143-145
|
C7H10N2
|
|
3.
|
179
|
60.33
|
7.26
|
23.46
|
8.93
|
161
|
C9H13N3O
|
|
4.
|
231
|
62.33
|
5.62
|
18.18
|
13.85
|
105-106
|
C12H13N3O2
|
Table 2: Mass And Ir Spectral Data Of The Compounds
|
Compound
|
Mass data m/z
|
IR Peaks (cm-1)
|
|
1.
|
137
|
3430,3111,3014,1668,1638,1602,1557,
1493,1336,1222
|
|
2.
|
122
|
3266,3090,3032,2998,1604,1576,1442,
1368,1312,1214
|
|
3.
|
164
|
3117,3107,3013,2963,1611,1588,1452,
1391,1322,1307,1228
|
|
4.
|
127
|
3269,3212,2000,1700,1669,1593,1453,
750,704
|
Table 3: 1H-NMR and 13C-NMR Spectral Data of the Compounds
|
Compounds
|
NMR Peaks (ppm)
|
|
1.
|
1H NMR δ 8.79(2H, m, H-5.6), 7.75(3H, m, H-2), 7.73(3H, m, H-3,4), 4.54(2H, d, J=3.7Hz, H-1),
13C NMR δ 163.95(C-1), 150.16(C-2), 140.27(C-3), 121.00(C-4).
|
|
2.
|
1H NMR δ 7.43(2H, dq, J=8.2,1.0Hz, H-4,5), 7.32(2H, m, H-6,8), 7.27(1H,m, H-7), 3.99(2H, dt, J=4.4,1.0 Hz, H-3), 2.80(1H, tt, J=4.4,3.3Hz, H-2), 2.09(2H, d, J=3.4Hz, H-1)
13C NMR δ 133.85(C-1), 129.35(C-2), 128.27(C-3), 128.00(C-4), 53.60(C-5).
|
|
3.
|
1H NMR δ 8.72(2H, dd, J=4.52Hz, H-4), 7.88(2H, dd, J=4.5Hz, H-3),
3.05(1H, dd, J=6.16,6.16Hz, H-2), 1.14(6H, d, J=6.16Hz, H-1)
13C NMR δ 164.35( C-1), 150.01(C-2), 134(C-3), 119.46(C-4),
58.06(C-5), 20.28 (C-6)
|
|
4.
|
1H NMR δ 7.316(1H, ddd, J=1.25,1.25Hz, H-1), 7.35(2H, ddd, J=7.71,7.79Hz, H-2), 7.317( 2H, ddd, J=1.27,7.71Hz), 3.914(2H, m, H-4), 6.55(1H, m, H-5), 2.554(3H,m, H-6)
13C NMR δ 169.9(C-1), 161.1(C-2), 153.8(C-3), 137.5(C-4), 128.9(C-5),
128.6(C-6), 127.79(C-7), 100.7(C-8), 49.3(C-9), 12.0(C-10)
|
Biological Activity
The biological activity of synthesized compounds are as follows.
Compound 1 and 3 showed antimycobacterial activity. This is checked by broth dilution method. Compound 1 and 3 shows antimycobacterial activity respectively at 191µg/ml and 176µg/ml.
Compound no. 3 and 4 shows antidepressant activity, compounds exert their action by inhibiting the enzyme monoamine oxidase(MAO). Inhibition results in increased levels of norepinephrine, dopamine, tyramine and serotonin in brain neurons and in various other tissues. For the assessment of antidepressant activity, Forced swim test (method) is used.
Compound 2 doesn’t shows any biological activity.
Results and Disscusion
Compounds were synthesized by amines, esters and arylhydrazides. These compounds shows anti TB(antimycobacterial) and antidepressant activity.
Aknowledgement
This work was supported by Govt. Dungar College and S.P. Medical College Bikaner.
References
- Majumdar P.,Pati A.,Patra M., Behera R. K.,Behera A. K. Chem. Rev. 2014;114:2942.
CrossRef
- Ling L., Urichuk L. J., Sloley B. D., Coutts R. T., Baker G. B.,Shan J. J.,Pang P. K. T. Bioorg.Med.Chem. Lett. 2001;11:2715.
CrossRef
- Èernuchová P.,Vo-Thanh G., Milata V., Loupy A, Jantová S.,Theiszová M. Tetrahedron. 2005;61:5379.
CrossRef
- Abadi A. H.,Eissa A. A. H.,Hassan G. S. Chem. Pharm. Bull. 2003;51:833.
CrossRef
- Tomasi A., Albano E. Toxicologic Pathology. 1987;15:178.
CrossRef
- Hearn M. J.,JooOn H. K.,Thai M. Bull. Soc. Chim. Belg. 1997;106:109.
- Sinha N., Jain S.,Tilekar A. ARKIVOC, (ii). 2005;9.
- Ling L., Urichuk L. J.,Sloley B. D., Coutts R. T., Baker G. B., Shan J. J.,Pang P. K. T. Bioorg. Med. Chem. Lett. 2001;11:2715.
CrossRef
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Ashry,E.H. Arch. Pharm. Chem. Life Sci., 339, 656 (2006).
CrossRef
- Danish, I.R.; Prasad, K.R., Acta Pharma., 54, 133-142 (2004).
- Manivel, P.; Roopan, S. M. J.Chil.Chem. Soc., 2, 2 (2009).
- Ragab, F. A.; Gawad, N. M. A.; Georgey, H. H.; Said, M. F., Eur. J. Med. Chem., 63, 645 (2013).
CrossRef
- Koopaei.M.N.; Assarzadeh, M.J. et al. Iranian J. of Phar. Resa., 12,721 (2013).
- Kadi, A. A.; El-Brollosy, N. R.; Al-Deeb, O. A.; Habib, E. E.; Ibrahim, T. M.; El-Emam, A.A., Eur. J. Med. Chem., 42, 235 (2007).
CrossRef
- Mogilaiah, K. and Sudhakar, G. R., Ind. J. Heterocycl. Chem., 11, 163 (2001).
- Demirbas, N.; Demirbas. A.; Karaoglu, S. A.; Celik, E., ARKIVOC, (i), 75 (2005).
- Farghaly, A. R.; El-Kashef, H., ARKIVOC , (xi), 76 (2006).
- Frahn,J.L.;Illman,R., J.Aust. J. Chem.,27, 1361 (1974).
CrossRef
- Hunsberger, M.; Shaw,E.; Fugger, J.; Ketcham,R.; Lednicer,D., J. Org. Chem. ,21, 394 (1956).
CrossRef
- Khan,K.M.;Rasheed,M.;Ullah,Z.;Hayat,S.; Kaukab,F.;Choudhary,M.I., Atta-ur- Rahman and Perveen,S., Bioorganic and Medicinal Chemistry ,11,1381 (2003).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Anil Gupta
and Anil Gupta Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal