An Investigation of Abrasion Resistance Property of Clay- Epoxy Nanocomposite Coating
Introduction
Organic inorganic hybrid nanocomposites show enhanced protective properties such as improvements in mechanical1,2 thermal3,4 optical5,6 electrical7 and magnetic properties.8 Polymer clay nanocomposites are material which consists of polymer as matrix and clay nanoparticles as the reinforcement fillers.9 Improved thermal, mechanical (strength, modulus, toughness) and physical (barrier) properties over pure nylon matrix were observed during initial research.10 Organically modified montmorillonite, hectorite and vermiculites are amongst the most commonly used fillers in polymer-clay nanocomposites.11 Enhancement in the barrier properties and corrosion protection by incorporation of SiO2, Zn, Fe2O3, halloysite clay nanomaterials were observed in epoxy coating.12,13 The novel polymer electrolyte nanocomposite composed of poly(ethylene oxide), lithium triflate and mineral clay study demonstrated that the addition of optimum content of D-2000 modified montmorillonite increased ionic conductivity of the PEO based electrolyte by nearly 16 times compared to plain (PEO)8 LiCF3SO3.14 Small improvement in the anticorrosion properties for the exfoliated SC18/Epon 828/D400 nanocomposite coatings was observed, however there were no improvement for the intercalated organoclay/Epon 828/Y-60 nanocomposite. This result might be related to the better dispersion of silicate nanosheet in the epoxy matrix for SC18/Epon 828/D400 nanocomposite.15 The processing and chemical characterization of amine cured epoxy/clay nanocomposites by FTIR and FT-Raman methods confirmed that the sonication processing in acetone did not induce chemical changes in the epoxy network. The intercalation of the epoxy network between the clay nanolayers was indicated by the broader tan δ curve observed by DMA. The Tg linearly increased with the increase of the amount of clay nanoplatelets in the amine-cured epoxy matrix.16 The incorporation of organoclay to rubbery and glassy epoxy matrices led to promising mechanical properties improvements even in the absence of additional shear to disperse the clay. With the use of organoclay, an interesting stiffness/toughness balance of nanocomposite for very low filler contents and without reduction in the Tg of the matrix was obtained.17 An advanced anticorrosive materials were prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties.18 Solvent assistance and curing at elevated temperature are main parameters for clay nanoparticles intercalation. Addition of small amounts of spherical nanoparticles with sufficient hardness such as zirconia nanoparticles assist the exfoliation process due to the shear implying media in nano-scale and fascinates the restoration of the physical and mechanical properties. Mechanical properties of nanocomposites containing various combination of clay and ZrO2 nanoparticles were slightly decreased compared with neat epoxy coating, This phenomena is as a result of formation of nano-sized voids in the trapped regions by clay stacks, and development of physical barrier clay stacks, which leads to disturb curing procedure and decrease polymer cross linking density. The barrier properties of the nanocomposites increased with the addition of spherical nanoparticles to clay nanocomposites due to better exfoliation process.19 Various combination of epoxy-based nanocomposite coatings containing aminopropyl trimethoxy silane, APS, treated zirconia and clay nanoparticles via slurry method were prepared. It was found that the simultaneous addition of layered clay and small amounts of spherical APS-treated zirconia nanoparticles with sufficient hardness to epoxy coatings significantly improves the corrosion resistance of the nanocomposite coatings.20 Epoxy coating modified by PANI/clay nanocomposite particles exhibited improved anticorrosion performance in comparison with neat epoxy and epoxy/PANI samples. Furthermore, the corrosion protection efficiency of steel panel coated with epoxy/PANI/OMMT (organo-modified montmorillonite) appeared to be much higher than that of epoxy/PANI/MMT one.21 The effects of different interface strength were significant on the morphology and structure of epoxy/clay nanocomposites. The XRD and SEM analyses proved that the chemical reaction of amino-groups of Jeffamine XTJ502-clay with epoxy created a better interface, resulting in significant mechanical properties enhancement.22 The increase in enthalpy of curing reaction and decrease in peak temperature of nanocomposite corroborated the catalytic effect of clay on the curing process. TEM and X-ray diffraction analysis confirmed both intercalated and exfoliated clay platelet within the epoxy blend.23 Two different procedures were used for the clay mineral dispersion: (i) organically modified montmorillonite (OMt) was dispersed in an epoxy matrix previously modified with acrylate polymers or (ii) the clay mineral was swollen in the acrylate monomers and the monomer was in situ polymerized followed by dispersion in the epoxy matrix. The later procedure provided higher degree of intercalation as indicated by small angle x-ray scattering (SAXS) and transmission electron microscopic (TEM) analysis. TEM microscopy was a very useful tool and indicated the presence of exfoliated/intercalated structures and some aggregates.24 The epoxy acrylate-clay nanocomposite prepared through the reaction of diglycidyl ether of bisphenol A (DGEBA) and acrylic acid in the presence of clay. The increase in surface hardness was observed which was found in agreement with the increase in glass transition temperature of the nanocomposite films.25 A new type of tetraglycidyl 1, 4′-bis (4-amine-phenoxy) benzene (TGBAPB) epoxy resin containing ether linkage was blended with conventional diglycidyl ether of bisphenol A (DGEBA) epoxy resin in order to overcome the deficient properties that prevented the use of DGEBA for high performance applications. Furthermore, organically modified montmorillonite nano clay of different weight percentages viz. 1%, 3% and 5% were reinforced with the TGBAPB/DGEBA blend epoxy to study its nano reinforcing effect. It was observed that thermal stability, mechanical properties, toughness and stiffness for the TGBAPB/DGEBA blend epoxy and nanocomposites are higher than that of neat DGEBA.26
Automotive, oil & gas pipelines, floorings etc. are important application segments for abrasion resistant polymeric linings as coating. In this article, we report abrasion resistance study of bentonite clay nanoparticles (b-CNPs)/ epoxy nanocomposite (ENC) coating with two different cure time intervals viz. one after 7 days and other after 60 days and compared it with neat epoxy (unfilled) coating.
Materials and Methods
Materials
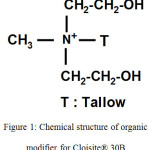
The clay nanoparticles (CNPs) in the current study is a methyl, tallow, bis 2-hydroxyethyl, quaternary ammonium modified bentonite having diffraction d-spacing (d) 18.5Å, obtained from BYK-Chemie GmbH, Germany commercially known as Cloisite 30B (chemical structure of the organic modifier is illustrated in Fig.1). Diglycidyl ether of bisphenol A (DGEBA) based unmodified medium viscosity liquid epoxy resin Araldite GY-250 obtained from Huntsman Corporation was used as polymer matrix. Amine curative used for crosslinking with epoxy is an aliphatic polyamine commercially known as Ancamine 1856, obtained from Air products, USA. A silicon based surface wetting additive (BASF) was added in small quantity (0.01%) in the coating formulation for surface tension reduction and smooth film formation. The epoxy resin, b-CNPs and surface additives were synthesized as ‘component A’ while aliphatic polyamine was used as ‘component B’ of the coating system. The Diglycidyl ether of bisphenol A epoxy polymer under study is having epoxide equivalent of 189 g/eq and viscosity of 12,000 cPs at 25°C while the curing agent used is having amine value 460 mg KOH/g and viscosity of 3000 Cps at 25°C.
Figure 1: Chemical structure of organic modifier for Cloisite® 30B
Synthesis of Clay Epoxy Nanocomposite (ENC) Coating
A 250 ml steel vessel was cleaned and dehydrated for 10 min in oven at 105°C. The vessel was charged with weighed quantity of Diglycidyl ether of bisphenol A epoxy polymer followed by silicon based wetting additive (0.01% by weight of total epoxy resin) and mixed for 5 minutes under mechanical stirrer (Dispermill, ATP Engineering B.V.). In this mixture, b-CNPs were charged to obtain hybrid nanoclay epoxy matrix composition in various desired concentration. A total of six hybrid epoxy matrix were synthesized following this method having weight concentrations 0.12%, 0.25%, 0.5%, 0.75%, 1% and 5% by high speed mechanical dispersion (HSD) method. The dispersion operations were performed at 1000 rpm, 30 minutes each batch following identical process of addition and similar dispersion level. Different dispersion methods have different effect on the clay modified polymers and non-identical dispersion may lead to different thermal, mechanical and performance properties.27 Maximum temperature recorded during the dispersion process was 45°C generated due to frictional force. Entire operation was performed at laboratory conditions, 23+/-1°C temperature and 55% relative humidity (RH).
Clay Epoxy Nanocomposites (ENC) Coating Properties and Concentrations
Various properties were studied at different concentrations of Diglycidyl ether of bisphenol A based epoxy clay nanocomposite coatings. Glass transition temperatures (Tg) and Fourier transform infrared spectroscopy (FTIR) tests were studied for 0% -5.0% CNPs concentrations. Scanning electron microscopy (SEM) were conducted for CNPs as such and ENC at 1% concentration to investigate the surface morphology. Abrasion resistance of the ENC were studied for 0% (neat) – 1.0% concentrations.
Organic–Inorganic Hybrid Nanocomposite Film Preparation
Thin films of epoxy CNPs formulation (component A) were prepared by spray and drawdown methods for various analyses and testing, after mixing in the ratio of 100:40 by weight with polyamine curative (component B). For abrasion resistance studies nanocomposite coatings were applied following gravity feed air assisted spray method on mild steel metal coupons of 100 mm x 100 mm x 1 mm dimension, using Devilbliss spray gun having 1.4 mm nozzle orifice. Abrasion resistance study for various concentrations were performed at 35 (±2) µ-meter dry film thickness (DFT). Before coating applications on mild steel coupons, the surfaces were prepared using emery paper of 180# followed by washing with acetone. Free films for scanning electron microscopy (SEM) analysis were prepared by draw down method on silicon release paper with calculated amount of material to have 25 µ-meter uniform dry film thicknesses. The applied films were kept at 23°C for 7 days before subjecting for test. Draw downed, dried composite films were used for thermal and spectroscopic studies.
Instrumental Characterization and Performance Testing
Different set of analyses and abrasion resistance property were studied for various concentrations of b-CNPs-ENC coatings. Thin films of clay doped Diglycidyl ether of bisphenol A epoxy coating materials were prepared by spray and drawdown methods for various analyses and testing. The abrasion resistance test of Cloisite®30B-Epoxy composite coatings was carried out according to ASTM D4060-01 method, for 0% (n-E), 0.12%, 0.25%, 0.5%, 0.75% and 1.0% concentrations. The carbon steel coupon surfaces were prepared using 3M aluminum oxide emery paper, 180# followed by washing with acetone before coating. The applied coatings/films were conditioned at 23°C for 7 days before testing. Draw downed, dried coating films were used for thermal and spectroscopic studies.
The thermal properties of the films were measured using differential scanning calorimetry (DSC) of TA Instruments, Inc. The weights of the samples were taken 10 milligrams each for thermal study of the composite coating. The glass transition temperature (Tg) of hybrid nanocomposite samples were measured heating from 25 to 200◦C at a heating rate of 20◦C/min under nitrogen atmosphere at a flow rate of 30 mL/min. The abrasion resistance test was carried out according to ASTM D4060-01 method. Scanning electron microscope (SEM) of JEOL-Tokyo model JSM-6510 LV was used to characterize the surface morphology of CNPs and ENCs film. Fourier transform infrared (FTIR) spectroscopic studies were done to investigate the interaction of b-CNPs with epoxy amine system in different concentrations using Thermoscientific Nicolet i-S10 instrument. Film applications for various types of specimen preparation under study were done by spray gun (Devilbliss, 1.4mm nozzle orifice) and draw down methods. The dry film thicknesses (DFT) of the films were measured by Elcometer – 456 thickness gauge.
Results and Discussions
Spectroscopic (FTIR) Studies of Cloisite®30B -ENC Composite Film
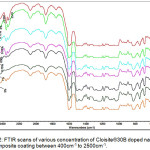
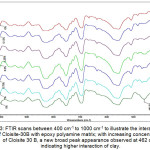
Fourier transform infrared spectroscopy (FTIR) method was adopted to confirm the changes in the chemical structures of the composite materials. To examine the possible interaction between the clay nano material and Diglycidyl ether of bisphenol A epoxy, a series of FTIR studies were carried out for various concentrations of b-CNPs-ENC coatings. The spectroscopic analysis was performed to analyze neat epoxy (n-E) and various concentrations of the epoxy polyamine nanocomposite coatings. The cured Cloisite®30B-epoxy polyamine nanocomposite (b-CNP-ENC) films were crushed to powder for this analysis. To closely visualize the spectral changes the spectra between 400cm-1 to 2500cm-1 are plotted as overlay for all the concentrations in Fig.2. Further, an enlarged portion of wavenumbers between 400 cm-1 to 1000 cm-1 has been plotted to distinctly visualize the spectral differences (Fig.3). Gradual enhancement in chemical interaction was observed with increase in Cloisite®30B concentration from 0.12% to 5% as corroborated by the peak intensity. A prominent peak was observed at wavenumber 462 cm-1 which shows interaction or inclusion of b-CNPs inside matrix of epoxy amine system. Different stretching/bending vibrations of chemical moieties in the infrared spectra observed during neat epoxy (0%) and epoxy NCCs have been given in Table.1. Two characteristic absorptions of the oxirane ring are observed in the range between 4000 cm-1 & 400 cm-1. The first one, at 915 cm-1, is attributed to the C-O deformation of the oxirane group, and the second band is located at 3050 cm-1 approximately and is attributed to the C-H tension of the methylene group of the epoxy ring. This band is not very useful since its intensity is low and it is also very close to the strong O-H absorptions; but in low polymerization degree epoxy monomers it can be used as a qualitative indicative of the presence of epoxy groups. The peaks were observed at 3400cm-1 for –OH group (moisture), 2360cm-1 for carbon dioxide, 1635cm-1 for –OH, 1384cm-1 for CO3 2- and 1170cm-1 for C-O. There are also bands corresponding to the ether linkage located at 1000-1100 cm-1. 28 It is noteworthy that b-CNPs are interacting with epoxy polyamine system and the intensity of the peaks at 462 cm-1 has been increasing with increase in concentration of Closite®30B from 0.12% to 5% concentrations. The initial interaction of the organo-clay with epoxy polyamine matrix is visible at 0.12% concentration with minor change in peak position and shift in peak intensity compared to n-E (0%). The changes at 0.25% concentration remained almost similar to the 0.12% concentration, a distinct peak of b-CNPs interaction appeared. Similar interaction with slightly increased intensity is visible at 0.75% and 1% concentrations. A very broad peak appeared with increase in concentration at 5% concentration which signifies the presence of Clositie®30B in the formulation.
Table 1: Spectral vibrations of different groups present in b-CNPs-ENC
|
Groups present
|
Wavenumber (cm-1)
|
|
b-CNPs interaction inside epoxy matrix
|
462
|
|
-CH, -NH Bend
|
557
|
|
-AR=C-H, C-H Bending out of plane (polyamide adduct)
|
754
|
|
1, 4- substitution of epoxy
|
828
|
|
-C-O-R Ether
|
1035
|
|
-C-N absorption of amine
|
1105
|
|
-C-N absorption of amine
|
1182
|
|
-C-C-O-C-Stretching
|
1248
|
|
Aromatic -C=C- H Vibration
|
1457
|
|
Aromatic -C=C- H Vibration
|
1508
|
|
Aromatic -C=C- H Vibration
|
1608
|
|
Ar-CH Overtones
|
1600-2000
|
Figure 2: FTIR scans of various concentration of Cloisite®30B doped nano composite coating between 400cm-1 to 2500cm-1.
Figure 3: FTIR scans between 400 cm-1 to 1000 cm-1 to illustrate the interaction of Cloisite-30B with epoxy polyamine matrix; with increasing concentrations of Cloisite 30 B, a new broad peak appearance observed at 462 cm-1 indicating higher interaction of clay.
Morphological (SEM) Characterization of b-CNPs-ENC film
The surface morphologies at various magnifications and scales of Cloisite®30B doped nanocomposite (b-CNPs-ENC) coating at 1.0% concentration are illustrated in Fig.4.1 A, B and C for 40x, 200x and 400x magnifications respectively. It is evident from the SEM images of b-CNPs-epoxy NCCs film that the clay particles are completely dispersed inside Diglycidyl ether of bisphenol A epoxy matrix with very smooth surface on top. This type of film smoothness which signifies uniformity in clay dispersion is expected to yield extremely high level of barrier property.
Figure 4: 1. SEM scan of Cloisite®30B-ENC, 1% concentration at 40x. [A]; 200x [B]; 400x [C], smooth surface finish, few bigger agglomerates distinctly visible at surface.
A few agglomerate of clay clusters measuring approximately 18, 21, 25, 23, 29 and 31 µ-meters are also visible on the surface, it is more distinctly visible in Fig.4.B. An enlarged view of the agglomerate has been shown in Fig.4.2 to distinctly visualize the agglomerate particles and their size. The reason of appearance of agglomerate might be attributed to the undispersed particles during mechanical dispersion process of b-CNPs inside Diglycidyl ether of bisphenol A epoxy matrix. The surface is smooth with no surface cracks, protrusion or non-uniformity visible in Fig.4.1.C. At 400x magnification, a few very small spread of b-CNPs are visible protruded from surface.
Figure 4: 2. Enlarged view of undispersed particles visualized in SEM image Fig. 4.1.B with measured particle size
Thermal (DSC) Characterization of Bentonite -ENC films
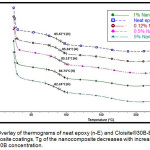
The glass transition temperature (Tg) of neat epoxy (n-E, 0%) and epoxy nanocomposite coatings (NCCs) in various concentrations viz. 0.12%, 0.5%, 1.0% and 5.0% were determined using differential scanning calorimeter (DSC) of TA Instrument model Q-20. The overlay of thermograms analyzed for various concentrations are given in Fig.5. The Tg is found to be shifting towards lower temperatures with increase in concentration of Cloisite®30B inside Diglycidyl ether of bisphenol A epoxy matrix observed in the thermogram. The lowering in the Tg of Diglycidyl ether of bisphenol A epoxy NCCs can be attributed to the non-cross linking with amine due to masking of reactive oxirane group by clay nanolayers inside polymer matrix. The lowering in Tg of nanocomposites has also been reported earlier in epoxy clay study by DSC analysis29 and by solid state NMR study.30 The glass transition temperature of the bentonite clay epoxy nanocomposite coating studied here in general, gradually decreasing with increase in clay material inside the epoxy matrix. It can be observed that at 0.12% and 0.50% concentration, there is almost negligible reduction in Tg while in case of 1% and 5% concentrations there is minor reduction when compared with neat epoxy coating, listed in Table.2.
Figure 5: Overlay of thermograms of neat epoxy (n-E) and Cloisite®30B-Epoxy nanocomposite coatings, Tg of the nanocomposite decreases with increase in Cloisite® 30B concentration.
Table 2: The glass transition temperature of b-CNPs-ENC
|
Cloisite 30B (b-CNP) concentration
|
0
|
0.0012
|
0.005
|
0.01
|
0.05
|
|
Glass transition temperature (Tg)
|
85.67
|
85.33
|
85.13
|
84.74
|
82.24
|
The finding on Tg in current research is supported by earlier research works that a slight increase or almost no change in Tg occurs in the systems with the organically treated clays modified by surfactants that bears polar group (-OH groups etc.).31,32,33,34 In those cases, where stronger interaction happens due to hydrogen bond between modifiers and epoxy, it might restrict the motion of epoxy network to some extent which in turn may result into higher Tg. The data of Tg states that in current system of b-CNPs-ENC coating, the nanolayers have not affected the thermal properties much up to 0.5% concentration, while at 1 and 5% concentrations, there might be minor reduction in crosslink density due to masking of reactive epoxide group of the coating making it softer hence, reduction in Tg.
Abrasion Resistance of Cloisite®30b-Enc Coating
The abrasion resistance test of the Cloisite30-Epoxy nanocomposite coatings were performed using Taber abrasion test method. The Taber abrasion test is used for the determination of the resistance of organic coatings to abrasion produced by the Taber Abraser on coatings applied to a metal panel. In current research work, the abrasion resistance values of nanocomposite coatings were determined by weight loss calculation method (method B) of the ASTM D4060 method.35 Abrasion resistance is calculated as loss in weight at a specified number of abrasion cycles, as loss in weight per cycle, or as number of cycles required to remove a unit amount of coating thickness.
Two different sets of specimen were prepared simultaneously using same material and similar application process for abrasion resistance study of b-CNPs-ENC coating. Coated specimen were tested at different time intervals to understand the effect of clay nanolayers inside Diglycidyl ether of bisphenol A epoxy matrix with cure time. The first set of experiment was performed after 7 days and that of second set after 60 days of curing. Both studies were performed after conditioning the test specimen in similar laboratory condition at 23+/-2°C temperature and 55% relative humidity (RH).
Abrasion of b-CNPs-ENC Coating after 7 days Curing
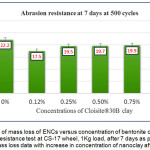
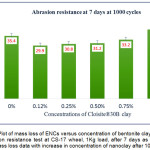
The abrasion resistance test conducted on 7 days cured film revealed that the Diglycidyl ether of bisphenol A epoxy – polyamine coating in unfilled condition (n-E, 0%) loses higher mass compared to Cloisite®30B doped ENC coating. Surprisingly, with mere introduction of b-CNPs at 0.12% concentration, the coating material demonstrated great enhancement in abrasion resistance property. The mass loss data for 7 days cured composites are plotted in Fig.6 and Fig.7 after 500 and 1000 cycles respectively. The best result was observed at 0.12% b-CNPs concentration for both the cycles.
Figure 6: Plot of mass loss of ENCs versus concentration of bentonite clay (b-CNPs) for abrasion resistance test at CS-17 wheel, 1Kg load, after 7 days as per ASTM D4060. A. mass loss data with increase in concentration of nanoclay after 500 cycles
Figure 7: Plot of mass loss of ENCs versus concentration of bentonite clay (b-CNPs) for abrasion resistance test at CS-17 wheel, 1Kg load, after 7 days as per ASTM D4060, mass loss data with increase in concentration of nanoclay after 1000 cycles.
It is noteworthy that the abrasion resistance result of 1% b-CNPs concentration observed a little poorer compared to n-E. The phenomena of improvement in abrasion resistance of the Diglycidyl ether of bisphenol A epoxy polyamine matrix with the introduction of bentonite clay might be attributed to higher compactness of the film due to clay filled Diglycidyl ether of bisphenol A epoxy, interaction of organically modified clay with Diglycidyl ether of bisphenol A epoxy amine matrix (reveled during FTIR study), shape and size of the clay particles and dispersion of particles inside the matrix. When compared among various concentrations, the higher abrasion resistance observed at lower concentration while gradual increase in concentration reduced the abrasion resistance from 0.25 to 1% concentration corroborates that with increase in the clay nanolayers inside matrix the bonding between organic moiety and inorganic clay decreased when cured for a limited period up to 7 days.
Abrasion Resistance b-CNPs-ENC Coating after 60 Days Curing
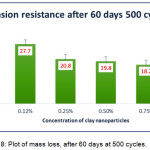
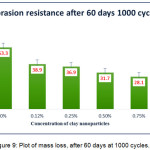
When tested after 60 days, again the trend of mass losses remains similar for both cycles 500 and 1000 illustrated in Fig.8 and Fig.9. A noteworthy difference observed in this case (compared to 7 days cured film) was with increase in concentration of b-CNPs, abrasion resistance keeps on increasing from n-E up to 0.75% and resulted very comparable to results at 1% concentration. From the Fig.8 and 9, it is obvious that the mass loss has decreased from 29.6 mg to 18.2 mg when tested to n-E and concentration of b-CNPs increased to 0.75% after 60 days exposure for 500 cycles. The decrease in mass loss compared to 7 days with extended curing period of 60 days indicates that the rigidity of the b-CNPs-ENC coating system has increased with time with increase in concentrations. Similarly, the specimen tested for 1000 cycles depicted least abrasion resistance at 0% (n-E) concentration, which got enhanced with b-CNPs doping. The mass loss reported for n-E is 53.3 mg while for 0.75% concentration it is 28.1 mg. The trend after complete cure is in agreement with FTIR interactions where gradual increase in peak observed with increasing intensity up to 0.75% concentration and depicted a very broad peak at 1% concentration. A minor decrease in abrasion resistance at 1% concentration shows that the b-CNPs are in excess for interaction with polymeric matrix. This is depicted in the FTIR scan for 1% with appearance of a very broad peak. 0.75% concentration has resulted as best concentration for abrasion resistance in current study.
Figure 8: Plot of mass loss, after 60 days at 500 cycles.
Figure 9: Plot of mass loss, after 60 days at 1000 cycles.
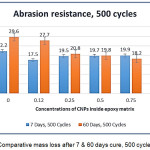
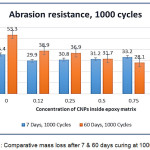
Comparative Analysis of b-CNPs-ENC Coating, 7 & 60 Days Curing
The comparative results of mass losses of 7 days and 60 days for neat epoxy (n-E, unfilled) depicts that it is having higher resilience in the chain after 7 days cure and illustrated higher abrasion resistance compared to that of tested after ageing for 60 days. The data of two separate cycles (500 and 1000) are plotted to understand how doping of b-CNPs behaves after 7 days and 60 days curing on abrasion resistance property. The plots of comparative mass losses are illustrated in Fig.10 & Fig.11. With introduction of b-CNPs inside Diglycidyl ether of bisphenol A epoxy matrix, enhancement observed in abrasion resistance even after 7 days. Also, after ageing 60 days, higher concentrations of b-CNPs have resulted into better resistance against abrasion which was not the case when tested after 7 days (0.12% performed best). It also implies that with time, the b-CNPs based nanocomposite film becomes more difficult to abrade out compared to neat Diglycidyl ether of bisphenol A epoxy. The enhancement in the abrasion resistance property can be attributed to gradual increase in the molecular interactions, film compactness due to inorganic b-CNPs filled inside matrix and lower movement in chain (higher rigidity) when completely cured thereby making it tougher for abrasion. It is also a resultant of interaction of clay particles with polymer matrix observed in FTIR analysis and platy shape with good dispersion inside Diglycidyl ether of bisphenol A epoxy matrix revealed by SEM analysis during current study. Higher interaction of clay nanoparticles for the properties improvements is in agreement with the earlier work reported by Jena KK, et al.36 Also, the decrease in mass loss with increase in the concentration of b-CNPs has been reported due to increase of inorganic materials inside polymer matrix37.
Figure 10: Comparative mass loss after 7 & 60 days cure, 500 cycles.
Figure 11: Comparative mass loss after 7 & 60 days curing at 1000 cycles
During current study it is noticed that approximately 0.75% concentration of modified bentonite CNPs (Cloisite 30B) is optimum for improvement and retention of abrasion resistance property of the currently designed coating system with time. The higher abrasion resistance coating so developed based on epoxy clay nanocomposite can have novel applications in various protective coatings systems including home and industrial floors (highly abusive area where conventional coatings show limited protection), mild steel pipes (wear happens during transportation), automotive engineering appliances etc. which undergo wear and tear with time.
Conclusion
Series of specimens of Diglycidyl ether of bisphenol A epoxy modified with organically treated bentonite clay nanoparticles (Cloisite®30B) were investigated for abrasion resistance property. No significant effect observed on glass transition temperature (Tg) with increase in concentration of b-CNPs inside epoxy matrix during current study which can be attributed to specific chemical nature of the nanoclay material and its interaction with Diglycidyl ether of bisphenol A epoxy amine matrix. It was noticed that with the increase in concentration of b-CNPs inside epoxy amine matrix decrease in mass loss occurs when abrasion resistance study was performed after 60 days cured specimen. Abrasion resistance of 0.75% concentration clay-epoxy composite coating found optimum during current study after 60 days ageing. The phenomena of higher abrasion resistance of the nanocomposites under current study may be attributed to increased rigidity, compactness and lower molecular mobility. Further, shapes, sizes and complete dispersion of clay nanolayers inside polymeric matrix (revealed by SEM images) depicted important contribution in enhancement of the abrasion resistance properties achieved. The improved abrasion resistance coating developed with incorporation of bentonite clay nano-particles can be applied for the protection of floors, pipes, automotive appliances etc.
Acknowledgment
One of the authors, Mukesh Kumar Madhup would like to acknowledge his employer 3M India Ltd. for providing permission for perusal of doctoral research at Gujarat University, Ahmedabad. Also, he is thankful to Dr. Sridhar Krishnamurthy, Mr. Abhijeet A. Saungikar, Mr. T. H. Venkatesh and Mr. Vijay G. Pillai for their continuous encouragement and support for higher study.
Conflict of interest: The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
Funding source :There is no funding source
References
- Wetzel B., Haupert F., Qiu Zhang M. Epoxy nanocomposites with high mechanical and tribological performance. Composites Science and Technology. 2003;63:14:2055-2067.
CrossRef
- Wang M., Fan X., Thitsartarn W., Chaobin H. Rheological and mechanical properties of epoxy/clay nanocomposites with enhanced tensile and fracture toughnesses. Polymer. 2015;58:43-52.
CrossRef
- Zheng K., Chen L., Li Y., Cui P. Preparation and thermal properties of silica-graft acrylonitrile-butadiene-styrene nanocomposites. Polymer Engineering & Science. 2004;44:6:1077-1082.
CrossRef
- Sushanta K. S., Mohanty S., Sanjay K. N. Study of thermal stability and thermo-mechanical behavior of functionalized soybean oil modified toughened epoxy/organoclay nano composite. Progress in Organic Coatings. 2015;88:263–271.
CrossRef
- Caseri W. Nanocomposites of polymers and metals or semiconductors: Historical background and optical properties. Macromolecular Rapid Communications. 2000;21(11):705-722.
CrossRef
- Li H., Wang D., Fan H., Wang P., Jiang T., Xie T. Synthesis of highly efficient C-doped TiO2 photocatalyst and its photo-generated charge-transfer properties. Journal of Colloid and Interface Science. 2011;354(1):175-180.
CrossRef
- Hu G., Zhao C., Zhang S., Yang M., Wang Z. Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer. 2006;47:1:480-488.
CrossRef
- Zhang M., Sun G., Guo D., Jin Z. Improvement of the magnetic properties of nanocomposite permanent magnetic alloys with Dy and Ga substitutions. Journal of Materials Processing Technology. 2002;120(1–3): 265-269.
CrossRef
- Fukushima Y., Okada A., Kawasumi M., Kurauchi T., Kamigaito O. Swelling behaviour of montmorillonite by poly-6-amide. Clay Minerals. 1988;23:1:27-34.
CrossRef
- Usuki A., Kojima Y., Kawasumi M., Okada A., Fukushima Y., Kurauchi T., et al. Synthesis of nylon 6-clay hybrid. Journal of Materials Research. 1993;8(05):1179-1184.
CrossRef
- Ray S. S., Okamoto M. Polymer/layeredsilicate nano composites a review from preparation to processing. Prog.Polym.Sci. 2003;28:11:1539–1641.
CrossRef
- Mei-Chun L., Kung-Chin C., Jui-Ming Y., Shir-Joe L., Ming-Fa H.,Herng-Show C. Advanced environmentally friendly anticorrosive materials prepared from water-based polyacrylate/Na+-MMT clay nano composite latexes.Eur. Polym. J. 2007;43(10):4219–4228.
CrossRef
- Shi X., Anh T. N., Suo Z., Liu Y., Avci R. Effect of nanoparticles on the anti corrosion and mechanical properties of epoxy coating Surf. Coat.Technol. 2009;204(3):237–245.
CrossRef
- Chen H. W., Chang F. C. The novel polymer electrolyte nano composite composed of poly (ethylene oxide), lithium triflate and mineral clay. Polymer. 2001;42:9763.
CrossRef
- Chen C., Khobaib M., Curliss D. Epoxy layered-silicate nanoc omposites. Prog. Org. Coat. 2003;47:376.
CrossRef
- Miyagawa H., Rich M. J., Drzal L. T. Amine-Cured Epoxy/Clay Nano composites. I. Processing and Chemical Characterization; J. Polym Sci Part B: Polym Phys. 2004;42:4384.
CrossRef
- Le Pluart L., Duchet J., Sautereau H. Epoxy/montmorillonite nan ocomposites: influence of organophilic treatment on reactivity, morphology and fracture properties.Polymer. 2005;46(26):12267.
CrossRef
- Hsiu-Ying H., Tsao-Cheng H .,Tzu-Chun Y., Chung-Yen T., Cheng-Ling L., Mei-Hui T.,Jui-Ming Y ., Yi-Chen C. Advanced anticorrosive materials prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties. Polymer. 2011;52(11):2391.
CrossRef
- Mirabedini S. M., Behzadnasab M., Kabiri K. Effect of various combinations of zirconia and organoclay nanoparticles on mechanical and thermal properties of an epoxy nano composite coating. Composites: Part A. 2012;43:2095.
CrossRef
- Behzadnasab M., Mirabedini S. M., Esfandeh M. Corrosion protection of steel by epoxy nano composite coatings containing various combinations of clay and nano particulate zirconia. Corro. Sci. 2013;75:134.
CrossRef
- Amir H. N., Joulazadeh M., Karimi F. Investigation of corrosion protection performance of epoxy coatingsmodified by polyaniline/clay nano composites on steel surfaces. Progress in Organic Coatings. 2014;77:347.
CrossRef
- Zaman I., Nor F., Manshoor B., Khalid A., Araby S. Influence of interface on epoxy/clay nano composites 1. morphology structure. Procedia Manufacturing. 2015;2:17.
CrossRef
- Sushanta K. Sa., Mohanty S., Sanjay K. N. Study of thermal stability and thermo-mechanical behavior of functionalized soybean oil modified toughened epoxy/organo clayna no composite. Prog. Org. Coat. 2015;88:263.
CrossRef
- Adriana A. S., Bluma G. S., Dahmouche K. Organoclay-epoxy nocomposites modified with polyacrylates: The effect of the clay mineral dispersion method. Applied Clay Science. 2016;124–125:46–53.
CrossRef
- Dehghan A.,Zohuriaan-Mehr M. J., Salimi A. Rapid preparation of epoxy acrylate-clay nano composite: Simultaneous acrylation/nanoclay dispersion under ultrasonication. Prog. Org. Coat. 2017;108:44.
CrossRef
- Duraibabu D., Rajagopal D., Ananda S. K. A first MMT reinforced nanocomposite functionalized with etherlinkage derived from tetraglycidyl/diglycidyl epoxy building block. Prog. Org. Coat. 2017;104:135.
CrossRef
- Adriana A. S., Bluma G. S., Dahmouche K. Organoclay-epoxy nocomposites modified with polyacrylates: The effect of the clay mineral dispersion method. Applied Clay Science. 2016;124–125:46–53.
CrossRef
- Madhup M. K., Shah N. K., Wadhwani P. M. Investigation of surface morphology anti-corrosive and abrasionresistance properties of nickel oxide epoxy nanocomposite (NiO-ENC)coating on mild steel substrate. Progress in Organic Coatings. 2015; 80:1–10.
CrossRef
- Akbari B., Bagheri R. Deformation mechanism of epoxy/clay nanocomposite. European Polymer Journal. 2007;43(3):782-788.
CrossRef
- Zax D., Yang D., Santos R., Hegemann H., Giannelis H., Manias E. J Chem Phys. 2000;112(6):1951-4.
CrossRef
- Messersmith P. B., Giannelis E. P. Synthesis and Characterization of Layered Silicate-Epoxy Nano composites. Chemistry of Materials. 1994;6(10):1719-1725.
CrossRef
- Miyagawa H., Rich M. J., Drzal L. T. Amine-cured epoxy clay nano composites. I. Processing and chemical characterization. Journal of Polymer Science Part B.Polymer Physics. 2004;42:23:4384-4390.
CrossRef
- Liu Z., Erhan S. Z., Xu J. Preparation, characterization and mechanical properties of epoxidized soybean oil/clay nanocomposites. Polymer. 2005;46:23:10119-10127.
CrossRef
- Chen K. H., Yang S. M. Synthesis of epoxy–montmorillonite nano composite. Journal of Applied Polymer Science. 2002;86(2):414-421.
CrossRef
- ASTM D4060-14, Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser, ASTM International, West Conshohocken, PA. www.astm.org. 2014.
CrossRef
- Jena K. K., Raju K. V. S. N., Narayan R., Rout T. K. Sodium montmorillonite clay loaded novel organic–inorganic hybrid composites: Synthesis and characterization. Progress in Organic Coatings. 2012;75(1–2):33-37.
CrossRef
- Guo Z., Pereira T., Choi O., Wang Y., Hahn H. T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties.Journal of Materials Chemistry. 2006;16(27):2800-2808.
CrossRef
Views: 1,500
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal

![Figure 4: 1. SEM scan of Cloisite®30B-ENC, 1% concentration at 40x. [A]; 200x [B]; 400x [C], smooth surface finish, few bigger agglomerates distinctly visible at surface.](http://www.materialsciencejournal.org/wp-content/uploads/2018/05/Vol15_No2_Inv_Muk_Fig4.1-150x150.jpg)