Microwave Assisted Phytosynthesis and Characterization of Magnetic Iron oxide Quantum Dots Using Moringa Oleifera
G. Deepthi Reddy1 , M. Noorjahan*1
, M. Noorjahan*1 , K.Girija Mangatayaru1
, K.Girija Mangatayaru1 and M.Krishnakanth2
and M.Krishnakanth2
1Department Of Chemistry, Palamuru University,Mahbubnagar, Telangana State-509001.
2Inorganic and Physical Chemistry Division, CSIR Indian Institute of Chemical Technology, Tarnaka, Hyderabad-500007.
Corresponding author Email: njan.hasanmohd@gmail.com
DOI : http://dx.doi.org/10.13005/msri/150206
Article Publishing History
Article Received on : 17-April-2018
Article Accepted on : 04-June-2018
Article Published : 13 Jun 2018
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Magnetic Iron Oxide Quantum Dots (MIOQDs) were synthesized using Moringa oleifera leaves through green technique i.e., Microwave treatment. The synthetic method is highly rapid, simple and economical. MIOQDs were formed by reduction of ferric chloride (FeCl3) solution with Moringa oleifera leaf extract without any influence of alkaline conditions which opens a new arena for the phytosynthesis of nanoparticles. MIOQDs structural characteristics were scrutinized by Powder X-ray diffraction method, FESEM, TEM, UV –Visible Spectroscopy, Photoluminescence Emission Spectroscopy. The ultraviolet-visible spectrum recorded for the aqueous media iron nanoparticles showed an absorption peak at around 330 nm. Powder X-ray diffraction showed that the particles are crystalline in nature, with both hematite and maghemite structure.
KEYWORDS:
Iron Oxide Quantum Dots; Moringa oleifera; Photoluminescence
Copy the following to cite this article:
Reddy G. D, Noorjahan M, Mangatayaru K. G, Krishnakanth M. Microwave Assisted Phytosynthesis and Characterization of Magnetic Iron oxide Quantum Dots Using Moringa Oleifera. Mat.Sci.Res.India;15(2)
|
Copy the following to cite this URL:
Reddy G. D, Noorjahan M, Mangatayaru K. G, Krishnakanth M. Microwave Assisted Phytosynthesis and Characterization of Magnetic Iron oxide Quantum Dots Using Moringa Oleifera. Mat.Sci.Res.India;15(2). Available from: http://www.materialsciencejournal.org/?p=7788
|
Introduction
Contemplation has been attained on the “Phytosynthesis” of nano metal oxides for their eminent optical, chemical, photochemical , and electronic properties.1 Nano Metal oxides, especially the noble metals, have mainly been studied due to their strong optical absorption in the visible region which will be caused by the combined excitation of free-electron gas.2 The phytosynthesis of nano metal oxidesimpersonates a relation between biotechnology and nanotechnology is usedto develop environmentally benign technologies for metal oxide synthesis which hasbeen received a great deal of attention now a days. The need of search for appropriate compatible biomaterials for the phytosynthesis of nano metal oxides continues through many different synthetic methods.3 By incredible physical, chemical, thermal , mechanical properties with suitable surface characteristics, nano metal oxideshave been widely used in cellular therapy, tissue repairs, targeted drug delivery, semi-conductor devices ,optics, electronics etc., Here and now, there are number of physical, chemical, biological, and hybrid methods available for synthesis of different types of nano metal oxides with varying structural morphologies and properties.4 The nano metal oxides formed using each method shows specific properties. However, phytosynthesis of nano metal oxides is currently under development.
Fabrication of nano metal oxides by phyto synthetic procedure is well suited for its spontaneous, economical, eco-friendly protocol involving onepot techniques for the synthesis process. Synthesis of nano metaloxides, mediated by the plants is influenced by the presence of phytochemicals. Phytochemicalssuch as flavonoids, terpenoids, carboxylic acids,quinones, aldehydes, ketones and amides are responsible for the spontaneous reduction of ions to form nano metal oxides.5
Iron Oxide nanoparticles such as Magnetite, Hematite, Maghamite have emerged as a distinct class of nanomaterials as they possess advanced properties like superparamagnetism, high coercivity etc ,useful in many bio-medical applications such as diagnosis, sensors, drug delivery and also as nano-sorbents in environmental engineering . Iron oxide nanoparticles have been mostly synthesized using different plant extracts by different green techniques such as sonochemical,solvothermal, hydrothermal synthesis. As from reported it is observed that 5-15 nm size that means low size iron nanoparticles were made known majorly from tea extracts as reducing agents.
In this study,we report a rapid Microwave assisted phytosynthetic method using hot extract of M.oleifera leaves for the synthesis of Magnetic Iron oxide Quantum Dots (MIOQDs) in ambient conditions, without any influence of surfactants. Moringa oleifera have many medicinal properties consisting poly phenolic compounds, flavonoids and glucosinates. 6,7which were engaged for the reduction and stabilizing of ions providesnanosized iron oxide materials with good optical and biological activity. In this work, the characterization of MIOQDs is discussed.
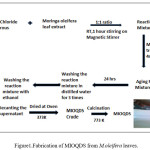
The MIOQDs were designed and synthesized using anhydrous ferric chloride as iron precursor and M.oleifera leaves as reducing agent and stabilizer.MIOQDs were characterized by XRD to know about the crystalline nature of particles, Fourier transform infrared spectroscopy (FTIR) for functional groups and, nature of the capping species, Transmission electron microscopy (TEM) for deep morphological observation,UV- visible spectroscopy to record the absorbance scanning in the range 200-1100nm and Photoluminescence spectroscopy in order to know the emission range of the MIOQDs by exciting at different wavelengths.
Materials and Methods
Chemicals and Equipment
Ferric chloride anhydrous (FeCl3 97%) was purchased from SDFCL , Mumbai , India and was used without further purification. Moringa oleifera leaves collected from local variety. Microwave MW73V with 2450 MHZ MW output, made in Malaysia was used for the synthesis of MIOQDS.
Preparation of the Extract solution
Moringa oleifera ( M.oleifera) leaves were collected from local variety. Twenty grams (20 g) of leaves were taken and washed with distilled water for 4 to 5 times in order to remove foreign particles ,then the leaves were crushed using mortar and pestle. The constituents in a beaker with distilled water(100ml), boiled for half an hour at 80oC. It was filtered through Whattmann filter paper and the supernatant was seized from the boiled constituents , Cooled at room temperature then the crude extract diluted with distilled water and stored at freezer in air tight bottles for further studies .
Synthesis of MIOQDs
Synthesis of MIOQDs has doneina 1:1 volume ratio ,adding 0.01 M FeCl3 solution to the hot extract of M.oleifera leaves. The pH of the mixture solution (200ml) is acidic (pH~6) and was
stirred at room temperature for 1hour, later the solution has given microwave treatment for 4minutes at 800W, hence MIOQDs were spontaneously obtained. The solution mixture was kept for aging for 24 hours then the mixture washed with water for thrice and with ethanol. The solution mixture was centrifuged and dried in oven at100 o C for 24 hours .Further it is calcined at 500 o C for 5 hours. The MIOQDs were collected for further use.
Initially, in the mixture solution,FeCl3 hydrolyzes to form ferric hydroxide and releases H+ ions thereafter, Ferric hydroxide gets partially reduced by the leaf extract constituents to form MIOQDS, while phenolic and other aldehyde groups in leaf are oxidized to the corresponding acids. The proposed phytosynthetic method for MIOQDs was found to be effective and irreducible.
Results and Discussion
The M.oleifera leaf extract containing polyphenols, flavanoids and glucosinates as a major constituents containing aldehyde,sulphate andhydroxyl groups may cause the reduction of Fe3+ shows the sudden change of color of solution mixture from pale yellow to brownish and stabilization of the MIOQDS14. While in the formation of MIOQDS, pHgets decreased which signifies the involvement of the OH- group in the reduction process. Initially, in the mixture solution,FeCl3 hydrolyzes to form ferric hydroxide and releases H+ ions thereafter, Ferric hydroxide gets partially reduced by the leaf extract constituents to form MIOQDS, thereafter on microwave treatment under moderate temperature of 4minutes time period produces mono dispersed Iron nanoparticles with good magnetic and structural properties. Microwave-assisted synthesis has a remarkable difference from the classic methods. Prominent advantage of microwave synthesis is the less reaction time and the homogenous heat supply causing almost immediate interaction of the electromagnetic field with the metal oxide particles being processed rather than surface heating which avoids agglomeration of metal oxide particles resulting size control of the nano metal oxides which is not effective and possible in room temperature. The photochemical reaction of formation of MIOQDS may be as:
M.oleifera leaves → OH– ions (may be from polyphenolic /flavanoid/glucosinate
FeCl3(s) → Fe+2(aq) +3 Cl–(aq)
n Fe+2(aq) + n OH– → FeOOH(aq)+ H+(aq)
FeOOH(aq) → α/ϒ- Fe2O3(s) + H2O
MIOQDS exhibits a supramagnetic nature in the presence of a external magnetic field.14 The iron oxide nano particles were tested in M.oleifera leaf extract by placing a magnet near the cuvette as shown in Figure1. The dark brownish MIOQDS being attracted by a magnet and when the applied magnetic force is removed, the magnetic nanoparticles can easily be dispersed by simple shaking. Thus, the magnetic nanoparticles can be separated with a simple magnetic device. The proposed phytosynthesis method for MIOQDS was found to be effective and irreducible.
The spectroscopic measurements of the colloidal dispersions of MIOQDS with the lower concentration (0.001M) were carried out with UV-1800 pc Shimadzu spectrophotometer in the wavelength region 200-1100 nm. The absorption spectrum recorded for MIOQDS synthesized by microwave irradiation treatment in the wavelength range 200-1100nm as shown in Figure 2.
Figure 2: UV-Visible spectrum of MIOQDs synthesized from M.oleifera leaf extract
The absorption wavelength range will predominate the probable color of solution.10,11 The spectra show a very low reflection from 400-500 nm and fine increase around 550 nm indicated that the band gap was around 2.2 eV. The weak absorption band in UV region from 200-400 nm were assigned to ligand to metal charge transfer between O(2p) orbitals and Fe3+ (d) orbitals and band in the visible region 600-700 nm results from spin-forbidden ligand field transitions.9
The phase formation of phytoreduced MIOQDs were studied with the help of XRD. The diffraction data of MIOQDS were recorded with X’pert Pro X-ray diffractometer (P analytical B.V., Netherlands) using Ni filtered Cu Kα radiation (λ= 1.5406Ao) from 2θ = 0–80 and operating at 40 kV and a current of 30 mA at a scan rate of 0.388 min−1 to determine the crystalline phase and structure. Figure 3 shows X-ray powder diffraction patterns at the ‘d’ values of 2.70, 2.51, 2.21, 1.84, 1.69, 1.48, 1.45, 1.42 Ao corresponds to rhombohedral α-Fe2O3 (Hematite,H) and ϒ- Fe2O3(Maghamite,M)[9]. ϒ-Fe2O3 particles have cubic unit cells with both octahedral and tetrahedral co-ordinated Fe3+ sites with defect spinel structures where as the unit cell of α-Fe2O3 is hexagonal and contain octahedrally co-ordinate Fe3+ sites.
Figure 3: XRD spectrum of biosynthesized MIOQDs containing Hematite-H and Maghamite- M
The X-ray diffraction confirms the presence of α-Fe2O3 and small amounts of ϒ- Fe2O3 phases12,13. The stability of spinel structure of ϒ- Fe2O3 relates to the smaller particle size and the defects and presence of the lattice disorder gives rise to typical optical properties of the Fe2O3 mixture.
The chemical composition of designed MIOQDS using M.oleifera leaves were characterized by FTIR spectroscopy using a Bruker Alpha FTIR spectrophotometer in order to know the organic moieties like aldehydes, alcohols etc.,present in the sample using KBr pellet method (wavenumber range 800-3600 cm-1) at room temperature. As shown in Figure 4,FTIR spectrum has shown well defined peaks at 561,1111, 1517, 1636 and 3426 cm-1 for synthesized iron oxide nanoparticles.
Figure 4: FTIR spectra of MIOQDs from M.oleifera extract
The emergence of sharp peak at 561 cm-1 is due to the presence of iron-oxygen bond(Fe-O)9,15 that confirms the particles synthesized are iron oxide nanoparticles. The appearance of a peak at 1111cm-1 is due to symmetric C-O vibration 8,14 .The band at 1517 cm-1 is due to presence of amide groups in glucosinates groups of M.oleifera leaves.7
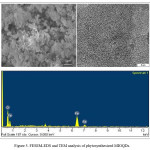
Figure 5: FESEM-EDS and TEM analysis of phytosynthesized MIOQDs
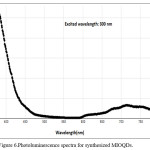
Photoluminiscence spectra of MIOQDS were recorded with Molecular Devices (SpectraMax M3) UV–Visible spectrophotometer operating at a resolution of 1 nm. The MIOQDS of lower concentration that is 1 mg in ethanol taken in cuvette and excited with a particular wavelength to know the emission spectra. The Pl curve recorded at a excited wavelength λmax=300 nm shows emission at 375 nm .In general the α-Fe2O3 shows an intense emission in the orange red region(Red-shift) of the spectrum.18 but in the present study of PL spectra , shows an intense emission peak in the ultraviolet region(Blue-shift)and broad emission peak in the visible region (Red-Shift).It is reported that PL spectra at different λ positions, ‘ λ’ of a sample is a function of the average diameter of the particles. As the particle size is small, recombination of electron-hole pairs is shifted to higher energies i.e., quantum confinement model.19 Thus, the PL emission in UV region is due to the smaller size of the Fe2O3 mixture as and emission in visible region is due to small portion of agglomerated nanoiron shown in Figure 6.
Figure 6: Photoluminescence spectra for synthesized MIOQDs
Conclusions
IOQDS were synthesized by phytoreduction of ferric chloride solution using a green fabrication technique with M.oleifera aqueous extract containing sulphated polysaccharides and polyphenolic compounds as the reducing agent. The hydroxyl, sulphate ,aldehyde and alcohol groups present in the leaf extract are apparently involved in the phytoreduction and stabilization of MIOQDS. The involvement of these groups in phytosynthesis is revealed by FTIR analysis. Structural analysis by surface morphology studies revealed synthesized particles are of average size below 5 nm. This exclusive and promising method of synthesizing MIOQDS could also be extended to fabricate other, industrially important nanometal oxides.
Acknowledgements
Equipment Facilities from Osmania University is praised for assistance with XRD. Fluorescence Spectroscopy facility from IICT Hyderabad and FESEM,EDS, TEM from National Dong Hwa University, Taiwan is appreciated. DST-INSPIRE (AORC) Fellowship, is gratefully acknowledged for financial support.
Conflict of Interest
There is no Conflict of interest between the authors.
Funding source
DST-Inspire has provided the funding for this research.
References
- Abhilash., Revati K.,Pandey B. D. Microbial synthesis of iron-based nanomaterials- A review.Bull.Mater. Sci. 2011;34:191–98.
CrossRef
- Guoa J.,Wanga J. R., Tjiu W. W., Pan J., Liu T. Synthesis of Fe nanoparticles@graphene composites for environmental applications.J. Hazard. Mater. 2012;225–226:63–73.
- Guptaa A. K., Gupta M. Synthesis and surface engineering of iron oxide nano particles for biomedical applications. Biomaterials. 2005;26:3995–4021.
CrossRef
- Liu J.,Qiao S. Z., Hu Q. H., Lu G. Q. Magnetic nano composites with mesoporous structures synthesis and applications. Small. 2011;7:425–443.
CrossRef
- Mihir H., Siddhivinayak B., Rakesh K. Review Article Plant-Mediated Green Synthesis of Iron Nanoparticles,Hindawi Publishing Corporation J. Nanoparticles. 2014;14:1-9. Article ID 1406.
- Gebregiorgis A. T. Chemical Compositions and Nutritional Value of Moringa Oleifera Available in the Market of Mekelle. J. Food and Nutrition Sciences. 2015;3:187-90.
CrossRef
- Ramesh k. S.,Sivanesan I., Keum Y. Phytochemicals of Moringa oleifera a review of their nutritional therapeutic and industrial significance.Biotech. 2016;6:1-14.
- Smaranika D., Umesh K. P., Birendra k. B. Green biosynthesis of Silver nano particles using Moringa oleifera leaf. Inter. J. Nanotech. Appl. (IJNA) . 2013;3:2:51-62.
- Hwang S .W.,Umar A., Dar G. N., Kim S. H., Badran R. I. Synthesis and Characterization of Iron Oxide Nanoparticles for Phenyl Hydrazine Sensor Applications.Sensor Letters. 2014;12:1–5.
CrossRef
- Tharani k., Nehru L. C., Synthesis and Characterization of Iron Oxide Nanoparticle by Precipitation Method Intern. J. Advanc. Res. Phys. Sci.(IJARPS). 2015;2:8:47-50.
- Morteza M., Abdolreza S., Imani M., Shokrgozar A. M.,Abbas S. M., Häfeli O. U., Pieter S. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic Iron oxide nanoparticles,Colloids and Surfaces B: Biointerfaces. 2010;5:300–309.
- Lu S. G., Bai S. Q., Xue Q. F. Magnetic properties as indicators of heavy metals pollution in urban topsoils: a case study from the city of Luoyang, China. Geophys. J. Int. 2007;171:568-580.
CrossRef
- Fahren N. S. ,Vivek P. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Nature.Scientific Reports. 2014;5:09733:1-9.
- Mahnaz M.,Farideh N., Bin A. M., Rosfarizan M. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum muticum) Aqueous Extract. Molecules. 2013;18:5954-5964.
CrossRef
- Andrade A. L.,Souza D. M.,. Pereira M. C., Fabris J. D., Domingues R. Z. Synthesis and characterization of magnetic nano particles coated with silica through a sol-gel approach. Cerâmica. 2009;55:420-424.
CrossRef
- Damon A. W.,Gongming W., Yichuan L., Yat L., Jin Z. Z. Nano structured hematite synthesis characterization, charge carrier dynamics and photo electro chemical properties. Energy Environ. Sci. 2012;5:682-6702.
- Sahoo S. K., Agarwal K., Singh A. K., Polke B. G., Raha K. C. Characterization of γ-and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3 ,Inter. J.Engin. Sci. and Tech. 2010;2:118-126.
- Saha S., Bhunia A. K. Synthesis of Fe2O3 Nanoparticles and Study of its Structural Optical Properties.J. Phys. Sci. 2013;17:191-195.
- Ledoux G., Gong J., Huisken F. Photo luminescence of size-separated silicon nano crystals Confirmation of quantum confinement, Appl. Phys.Lett. 2002;80:4834-4836.
CrossRef
Views: 1,624
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , M. Noorjahan*1
, M. Noorjahan*1 , K.Girija Mangatayaru1
, K.Girija Mangatayaru1 and M.Krishnakanth2
and M.Krishnakanth2
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal