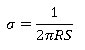Infrared spectra: Useful Technique to Identify the Conductivity level of Emeraldine form of Polyaniline and Indication of Conductivity Measurement either Two or Four probe Technique
Introduction
The oxidation state. The tunable electrical conductivity accomplished by doping/de-doping makes PANI is a promising material for some, applications incorporating into batteries, sensors, actuators, electromagnetic Polyaniline is most studied conducting polymer throughout the world because of its easy synthesis, environmental stability, reasonably good electrical conductivity, tunable electrical properties via oxidation/reduction chemistry (unique acid/base doping/dedoping process), and inexpensive material. PANI exists in three different forms, i.e., leucoemeraldine (completely insulator), emeraldine (insulator to the semiconductor) and pernigraniline (conductive). Polyaniline can be synthesized by chemical oxidative synthesis,1 electrochemical polymerization,2 rapid mixing polymerization,3 Emulsion Polymerization,4 in situ seeding polymerization5 or using templates and surfactant.6 Among these techniques, interfacial polymerization is a standout amongst the best methodologies, which creates amazing PANI nanostructures in extensive quantities.7 The conductivity of polyaniline emeraldine base (PANI-EB) increments reversibly with doping from the un-doped base (10-10 S cm-1) to the completely doped, emeraldine directing salt frame (101 S cm-1). The doping level can be tuned basically by controlling the pH of the dopant and this can be controlled either chemically or electrochemically by changing protecting, antistatic coatings, corrosion resistance, electro-optic, electrochromic gadgets, and division layers and so on. Synthesis, properties, and uses of emeraldine type of PANI frameworks have been accounted in the reviews.8-10
PANI is typically prepared in water medium at room temperature11 or at low temperature.12,13 The response continues to the development of a green encourage. PANI can likewise be set up in the solid state when no dissolvable is used14,15 for the two strategies just granular morphology is typically framed. Then again, if polymerization continues at the interface of two immiscible fluids, PANI nanofibres are obtained.16,17 On the off chance that amid oxidation a surfactant or a water-solvent polymer is added to the reaction, PANI colloids are shaped.
It has been exhibited by Gospodinova et al.,18 that the polymerization of aniline additionally continues well in water, with no additional acid, when ammonium peroxydisulfate was utilized as an oxidant. In our gathering likewise already revealed that the aniline was oxidized with APS at high temperature with no acid dopant.19
In the present work, PANI-ES were set up without utilizing any additional hazardous acid by the oxidation of aniline utilizing APS. PANI-ES was de-doped to PANI-EB utilizing aq. NaOH. In the present paper, aniline was oxidized with ammonium peroxy di sulfate in water/organic solvent medium without any additional acid, and was evaluated by morphology, FT-IR and conductivity measurements. The conductivity of the sample >0.1 S cm-1 to be estimated by means of four probe technique, and the conductivity <10-9 S cm-1 to be estimated utilizing high resistance meter. Conductivity in the range 10-2 to 10-9 S cm-1 can be estimated utilizing even a typical multimeter. Four probe procedures include the estimation of voltage by passing the current. Subsequently, it needs consistent current source and voltage estimation hardware with the required exactness, which are costlier gear for the business. This paper reports that FT-IR could be utilized to discover the conductivity level in emeraldine frameworks, and the strategy for estimation of the conductivity of the emeraldine samples through high resistance meter, two or four probe methods.
Polyaniline-sulfate salt (PANI-H2SO4)20 was prepared by traditional polymerization pathway by the oxidation of aniline within the sight of sulphuric acid utilizing ammonium persulfate oxidant (APS).
Materials and Methods
Materials
Aniline (AR grade) was obtained from S. D. Fine Chemicals, Mumbai, India was vacuum distilled prior to use. Ammonium persulfate and Sulphuric acid (H2SO4) were obtained from Sigma Aldrich, Bengaluru, India. All the solvents (AR grade) were obtained from Rankem, Hyderabad, India. All the reactions were carried out with distilled water. Polyaniline-sulfuric acid (PANI-H2SO4) salt and its corresponding base were prepared by following our earlier report.20
Characterization
The details of characterization techniques are given in Table 1.
Table 1: Techniques used along with information and their equipment details
|
Technique
|
Condition
|
Equipment details
|
|
Fourier-transform infrared spectroscopy (FT-IR)
|
Sample was evenly dispersed in Potassium bromide (KBr) by grinding and pressed into pellet
|
Model 670, Nicolet Nexus, (Minnesota, USA)
|
|
Scanning electron microscopy (SEM)
|
Powder samples
|
Hitachi S-4300 SE/N FE-SEM, (Hitachi, Tokyo, Japan)
|
|
Two-probe conductivity measurement
|
Pellet (13 mm diameter and 1.5 mm thickness)
|
Keighley, Cleveland, OH, model-2010
|
|
Four-probe conductivity measurements
|
Pellet (13 mm diameter and 1.5 mm thickness)
|
Keighley, Cleveland, Ohio, Current source-6220 and nanovoltmeter -2182A
|
Experimental Procedure
Synthesis PANI-ES in organic solvents without using acid dopants
A progression of polyaniline emeraldine salts (PANI-ES) incorporated with the nonappearance of corrosive through interfacial polymerization4 of aniline in water and natural dissolvable blend. In an ordinary test,15 mL of solvent was taken in a 100 mL tapered flagon and included 1 mL of aniline. An oxidizing specialist APS (2.8 g) in 30 mL of water was added gradually through the side of the funnel shaped flagon amid a time of 15 to 20 min. The response blend was kept at 5 oC in an icebox without mixing for 24 h. The green shaded powder was separated, comprehensively flushed with water refined lastly with 250 mL of (CH3)2CO. The powder test dried at 60 oC till a steady weight.
In this combination of PANI-ES utilizing two arrangements of solvents, i.e., First arrangement of solvents comprising of non-polar solvents, i.e., xylene, toluene, benzene, hexane, dichloromethane (DCM), chloroform and the second arrangement of solvents are polar solvents, i.e., water, methanol, ethanol, isopropyl alcohol (IPA), tetrahydrofuran (THF).
Preparation of PANI-EB
De-doped the polyaniline emeraldine salt (PANI-ES) to polyaniline emeraldine base (PANI-EB) by blending 0.5 grams of PANI-ES in 50 mL of an aq.1 M NaOH for 4 h at enveloping temperature. The blend was isolated and washed with 50 mL of aq.1 M NaOH, 500 mL of deionized water, in conclusion, 50 mL of (CH3)2CO. The powder was dried at 60 °C until to obtain a constant weight.
Results and Discussions
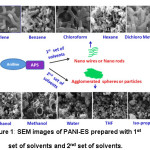
Morphologies of the PANI-ES prepared in the mixture of aqueous/organic solvents medium are shown in Fig.1. The images showed mostly two types of morphologies, i.e. nanowires or nanorods and agglomerated spheres or particles.
Figure 1: SEM images of PANI-ES prepared with 1st set of solvents and 2nd set of solvents.
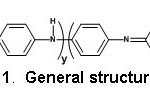
FTIR spectra and conductivity measurements were carried out for the PANI-ES and their corresponding bases. The PANI-EB can, in principle, be described by the following general structure (Structure 1).
Structure 1: General structure of PANI-EB.
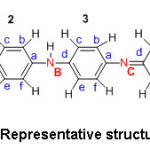
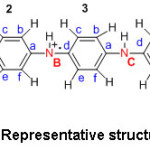
In the summed up base frame, (1-y) measures the capacity of oxidized units. At the point when (1-y) = 0, polymer has no such oxidized gathering and is ordinarily known as a leucoemaraldine base. The completely oxidized frame, (1-y) =1 is alluded to as a pernigraniline base. The half-oxidized polymer where the quantity of reduced units and the oxidized units are equivalent, i.e. (1-y) = 0.5, is of exceptional significance and is named the emeraldine oxidation state or the emeraldine base. The estimation of ‘y’ changes from 0 to 1 at the same time, the level of carbon; hydrogen and nitrogen percentage is nearly the same. Contemplating the above focuses, the accompanying structures for emeraldine base and emeraldine salt are considered for the present talk (Structures 2 and 3).
Structure 2: Representative structure of PANI-EB.
Structure 3: Representative structure of PANI-ES.
Major peaks are analyzed by considering the above structure of PANI-ES and PANI-EB. PANI-EB has FT-IR functional groups such as N-H, C=C, C C, C-C, C-N-C, C C-H, C-N=C, 1, 4-disubstituted benzene (NC-C6H4-ND). Peaks due to these functional groups are obtained in the FT-IR spectrum of PANI-EB is shown in Table 2.
Table 2: Infrared spectral data of PANI-H2SO4 salt and its corresponding base along with the reported PANI salts and their bases
|
System
|
NA-H d
|
NB–H+●
|
C=C
4b-4c
|
C C
1b-1c
|
C-C str.
4c-4d
|
C-N-C
1a-NA-2d
|
C C-H
4b-H
|
C-N H+●-C
3a-ND-4d
|
NC-C6H4-ND H+●.
di sub.
|
|
PANI-H2SO4a
|
3440
|
3225
|
1563
|
1482
|
NIL
|
1300
|
1240
|
1105
|
801
|
|
PANI basea
|
3380
|
NIL
|
1585
|
1494
|
1380
|
1307
|
1213
|
1160
|
828
|
|
Reported PANI Salts b
|
3440
|
3225
|
1560-
1570
|
1470-
1484
|
NIL
|
1298-
1306
|
1230-
1243
|
1105-
1130
|
797-803
|
|
Reported PANI Basesc
|
3440
|
NIL
|
1585-
1590
|
1492-
1500
|
1377-
1383
|
1310-
1313
|
1213-
1217
|
1159-
1162
|
824-835
|
a PANI-H2SO4 salt and its base prepared from our earlier report [20], b Ref No. [21-24],c Ref No. [19, 21, 25], d Very broad peak
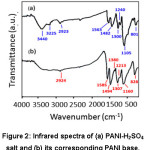
On protonation, PANI-EB changes to PANI-ES, wherein, nitrogen atom changes to protonated radical cation (NH+●). This PANI-ES has the functional groups such as N-H, N-H+●, C=C, C C, C-N-C, C C-H, C-NH+●-C, 1, 4-disubstituted benzene (NC-C6H4-NDH+●). Thus in the case of PANI-ES, an extra peak should appear for N-H+● (3225 cm-1), and the peak due to C-C (1380 cm-1) disappear when compared with that of PANI-EB. Also, peak shifts are expected due to the aromatic nature of PANI-ES. These changes are also observed in the PANI-H2SO4 salt (Table 2). In addition, based on the present result and experience of the authors, the range of the peaks for most of the PANI-salts and bases were identified. These values are also included in Table 2. FT-IR spectra of PANI-H2SO4 salt and its base are shown in Fig. 2 and the major peaks observed are indicated in Fig. 2.
Figure 2: Infrared spectra of (a) PANI-H2SO4 salt and (b) its corresponding PANI base.
The FT-IR spectral results for the PANI-ES prepared using various solvents are detailed in Table 3. These PANI-ES spectra showed peaks due to N-H, N-H+●, C=C, C C, C-N-C, C C-H, C-NH+●-C, 1, 4-disubstituted benzene, this signs indicate the formation of PANI in salt form.
Table 3: Infrared spectral data for PANI-ES salts prepared using 1st and 2nd set of solvents
|
Emeraldine
Salts
|
C=C
4b-4c
|
C C
1b-1c
|
C-C str.
4c-4d
|
C-N-C
1a-NA-2d
|
C C-H
4b-H
|
C-NH+●-C
3a-ND-4d
|
NC-C6H4–DH+●.
di sub.
|
Conductivity
(S cm-1)
|
|
1st Set of solvents
Xylene
|
1565
|
1481
|
s
|
1300
|
1244
|
1105
|
799
|
1.5
|
|
Toluene
|
1567
|
1484
|
NIL
|
1301
|
1242
|
1108
|
799
|
0.8
|
|
Benzene
|
1567
|
1486
|
NIL
|
1296
|
1244
|
1106
|
797
|
0.3
|
|
Hexane
|
1567
|
1484
|
NIL
|
1298
|
1245
|
1107
|
799
|
0.9
|
|
DCM
|
1563
|
1480
|
NIL
|
1300
|
1241
|
1104
|
797
|
0.3
|
|
Chloroform
|
1560
|
1482
|
NIL
|
1300
|
1242
|
1105
|
799
|
0.4
|
|
2nd Set of solvents
Water
|
1574
|
1497
|
vs
|
1306
|
1260
|
1147a
|
823
|
0.009
|
|
Methanol
|
1584
|
1498
|
vs
|
1301
|
1260
|
1145a
|
822
|
0.02
|
|
Ethanol
|
1569
|
1492
|
vs
|
1303
|
1255
|
1113a
|
821
|
0.02
|
|
IPA
|
1569
|
1499
|
vs
|
1293
|
1260
|
1116a
|
823
|
0.0007
|
|
THF
|
1579
|
1493
|
vs
|
1302
|
1260
|
1112a
|
820
|
0.0007
|
vs – Very small peak observed, s is small a peak appears as doublet peak
FT-IR spectral results for the PANI-EB prepared from various PANI-ES synthesized using various solvents are detailed in Table 4.
Table 4: Infrared spectral data for PANI-EB
|
Emeraldine
base
|
C=C
4b-4c
|
C C
1b-1c
|
C-C str
4c-4d
|
C-N-C
1a-NA-2d
|
C C-H
4b-H
|
C-NH+●-C
3a-ND-4d
|
NC-C6H4-NDH+●.
di sub.
|
|
1st Set of solvents
Xylene
|
1589
|
1502
|
1378
|
1309
|
1228
|
1164
|
830
|
|
Toluene
|
1590
|
1502
|
1379
|
1314
|
1229
|
1162
|
829
|
|
Benzene
|
1590
|
1502
|
1380
|
1307
|
1228
|
1161
|
833
|
|
Hexane
|
1590
|
1502
|
1380
|
1312
|
1228
|
1164
|
832
|
|
DCM
|
1588
|
1502
|
1380
|
1311
|
1228
|
1163
|
828
|
|
Chloroform
|
1587
|
1502
|
1379
|
1312
|
1229
|
1164
|
830
|
|
2nd Set of solvents
Water
|
1591
|
1504
|
1378
|
1310
|
1229
|
1166
|
834
|
|
Methanol
|
1589
|
1488
|
1379
|
1310
|
1228
|
1160
|
830
|
|
Ethanol
|
1588
|
1500
|
1379
|
1302
|
1228
|
1164
|
829
|
|
IPA
|
1585
|
1496
|
1379
|
1294
|
1228
|
1165
|
834
|
|
THF
|
1590
|
1502
|
1380
|
1304
|
1228
|
1166
|
830
|
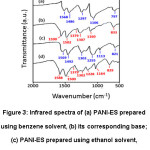
Most of the FT-IR peaks of PANI-EB are shifted compared to the PANI-ES peaks due to the formation of anilinium radical cation (N-H+●) in PANI-ES. As representative systems, FT-IR spectra of PANI-ES prepared using benzene (1st set of solvent) and ethanol (2nd set of solvents) and their corresponding bases in the range 2000-500 cm-1 are shown in Fig. 3 along with their major peak positions.
Figure 3: Infrared spectra of (a) PANI-ES prepared using benzene solvent, (b) its corresponding base; (c) PANI-ES prepared using ethanol solvent, and (d) its corresponding base.
Two sets of results observed based on the solvents used. The first set of solvents consisting of xylene, toluene, benzene, hexane, dichloromethane, chloroform and the second set of solvents are water, methanol, ethanol, isopropanol, tetrahydrofuran. The peaks observed at lower wavenumber for the PANI-ES prepared using the first set of solvents compared to that of the PANI-ES prepared using the second set of solvents. In addition, a peak appears in the case of the second set of solvents at around 1040 cm-1 and this peak is due to C-O-C. The clear peak shifts are observed in C=C (quinonoid ring), C C (benzenoid ring), C-NH+●-C and NC-C6H4-NDH+●.di substituted benzene. The range of FT-IR peaks observed for reported PANI-salts, PANI-ES prepared using various solvents and its corresponding bases are listed in Table 5.
Table 5: Correlation between FT-IR peaks with conductivity and its measurement technique
|
System
|
C=C
4b-4c
|
C…C
1b-1c
|
C-NH+●-C
3a-ND-4d
|
NC-C6H4-NDH+●.
di sub.
|
Conductivity
(S cm-1)
|
Resistance
Measurement
|
|
Reported
PANI salts a
|
1560-1570
|
1470-1484
|
1105-1130
|
797-803
|
3 to 5
|
Four probe
|
|
PANI-ES
(1st set of solvents) b
|
1560-1567
|
1481-1486
|
1105-1108
|
797-799
|
0.3 to 1.5
|
Four probe
|
|
PANI-ES
(2nd set of solvents) b
|
1569-1584
|
1492-1499
|
1112-1145
|
820-823
|
0.02 to 0.0007
|
Two probe
|
|
Bases from
PANI salts prepared in various Solvents b
|
1585-1590
|
1496-1504
|
1160-1165
|
828-834
|
< 10-10
|
High resistance
Meter
|
aRef No.[21-24], b Prepared in this work
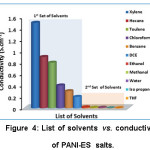
This Table 5 also indicates the correlation between FT-IR spectrums of PANI systems with its conductivity and method of measurement of conductivity of PANI systems. This information is a very useful researcher and industrialists working on PANI systems to find the conductivity level and method of measurements from FT-IR spectrum. Similarly, the conductivity of PANI-ES shows two sets of value (Fig. 4), i.e., >0.3 S cm-1 for the first set of solvents and <0.3 S cm-1 for the second set of solvents.
A clear correlation observed between the conductivity and FT-IR peak positions for emeraldine salt and its base. Reported emeraldine polyaniline salts having conductivity (2 to 5 S cm-1) showed peaks due to C=C, C C, C-NH+●-C and NC-C6H4-NDH+●. at lower wavenumbers compared to its corresponding base, which is having very low conductivity (<10-10 S cm-1). PANI-ES prepared using first set of solvents are showing conductivity >0.3 S cm-1, which showed FT-IR peaks close to that of PANI-H2SO4 salt peaks.
Figure 4: List of solvents vs. conductivity of PANI-ES salts.
The second set of solvents PANI-ES showed conductivity in the semiconductor region, which showed peaks in between the emeraldine salt and its base. In a similar manner, PANI-ES prepared using first set of solvents with conductivity (>0.3 S cm-1) shows nanowires or nanorods morphology and the PANI-ES prepared with the second set of solvents with lower conductivity (<0.3 S cm-1) shows agglomerated spheres or particles morphology.
Conductivity measurement by four-probe method on polymer pellets by the following equation.
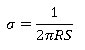
Here, the distance between the two points is equal. Where,σ is Conductivity in S cm-1, R=I⁄V, I is the current flowing through the sample, V is produced the voltage across two inner points, and S is the probe spacing about ~0.2 mm.
Conductivity measurement by a two probe method by the following equation
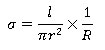
Where l is the thickness in cm, r is the radius in cm and R is the resistance of the pellet in Ω.
Morphology and conductivity are important phenomenal in PANI systems. In this work, we notified morphologies and conductivity tuning with solvents. These results in signpost solvent choosing also important factor prepare of emeraldine PANI systems.
Conclusion
In conclusion, emeraldine form of PANI either in salt or base form could be found from the FT-IR spectrum without conductivity measurement. In this work, PANI-ES systems were prepared without acid in two sets of solvents. A correlation between FT-IR spectral peaks and conductivity level of emeraldine PANI system was established. Also, FT-IR spectral peaks could be used to indicates the type of method of conductivity measurement i.e., either two probe or four probe techniques. The peaks observed at lower wavenumber in FT-IR spectrum for the higher conductivity PANI-ES (>0.3 S cm-1) compared to the lower conductivity PANI-ES (<0.3 S cm-1). The scope of this work is to indicate that FT-IR spectrum could be used to find out the conductivity level of PANI-ES samples and its measurement technique, i.e. either using ordinary multimeter with two probes or four probes measurement.
Acknowledgment
We acknowledge CSIR-IICT for financial support, Project No. (MLP-0006). G.R acknowledge to CSIR, India for financial support.
Funding Source
We thank CSIR-IICT for financial support, Project No. (MLP-0006). G.R acknowledge to CSIR, India for financial support.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- A. Moyseowicz, G. Gryglewicz, Hydrothermal-assisted synthesis of a porous polyaniline/reduced graphene oxide composite as a high-performance electrode material for supercapacitors, Compos Part B-Eng. 159, 4-12 (2019).
CrossRef
- Y. Wu, J. Wang, B. Ou, S. Zhao, Z. Wang, S. Wang, Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance. Nanomaterials. 8, 103 (2018).
CrossRef
- J. Deng, J. Guo, P. Liu, Growth of polyaniline nanomaterials in rapid-mixing polymerization, Colloids and Surfaces A: Physicochemical and Engineering Aspects. 521, 247-250 (2017).
CrossRef
- G. Ramesh, S. Palaniappan, D. D. Lac, S. V. Bhosale, Improving the photocatalytic activity of polyaniline and a porphyrin via oxidation to obtain a salt and a charge-transfer complex. New. J. Chem. 41, 14595-14601 (2017).
CrossRef
- X. Zhang, J. W. Goux, S. K. Manohar, Synthesis of Polyaniline Nanofibers by “Nanofiber Seeding, J. Am. Chem. So. 126, 4502-4503 (2004).
CrossRef
- L. Yu, J. I. Lee, K. W. Shin, C. E. Park, R. Holze, Preparation of aqueous polyaniline dispersions by micellar-aided polymerization. J. Appl. Polym. Sci. 88, 1550-1555 (2003).
CrossRef
- Z. Fanxin, Q. Zongyi, L. Banglei, L. Tao, L. Na, Z. Meifang, Polyaniline nanostructures tuning with oxidants in interfacial polymerization system. Prog. Nat. Sci. Mater. 25, 512-519 (2015).
CrossRef
- Z. A. Boeva, V. G. Sergeyev, Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C. 56, 144-153 (2014).
CrossRef
- S. Palaniappan, A. John, Polyaniline Materials by Emulsion Polymerization Pathway. Prog. Polym. Sci. 33, 732-758 (2008).
CrossRef
- C. M. Gordana, Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 177, 1-47 (2013).
CrossRef
- J. Stejskal, R. G. Gilbert, Polyaniline, Preparation of a conducting polymer, Pure Appl Chem. 74, 857-867 (2002).
CrossRef
- J. Stejskal, M. Spirkova, A. Riede, M. Helmstedt, P. Mokreva, J. Prokes, Polyaniline dispersions 8. The control of particle morphology. Polymer. 40, 2487-2492 (1999).
CrossRef
- S. Chen, X. Zhang, B. Liu, H. Shi, F. Chen, C. Hu, J. Chen, Characterisations of carbon-fenced conductive silver nanowires-supported hierarchical polyaniline nanowires. Electrochim. Acta. 292, 435-445 (2018).
CrossRef
- O. Y. Posudievsky, O. A. Goncharuk, V. D. Pokhodenko, Mechanochemical preparation of conducting polymers and oligomers. Synth. Met. 160, 47-51 (2010).
CrossRef
- O. Y. Posudievsky, O. A. Goncharuk, R. Barille, V. D. Pokhodenko, Structure–property relationship in mechanochemically prepared polyaniline. Synth. Met. 160, 462-467 (2010).
CrossRef
- S. Xing, H. Zheng, G. Zhao, Preparation of polyaniline nanofibers via a novel interfacial polymerization method. Synth. Met. 158, 59-63 (2008).
CrossRef
- N. V. Blinova, M. Trchova, J. Stejskal, The polymerization of aniline at a solution–gelatin gel interface. Eur. Polym. J. 45, 668-673 (2009).
CrossRef
- N. Gospodinova, P. Mokreva, L. Terlemezyan, Chemical oxidative polymerization of aniline in aqueous medium without added acids. Polymer. 34, 2438-2439 (1993).
CrossRef
- S. Palaniappan, S. Balsydulu, P. Lakshmi, P. Srinivas, High-temperature oxidation of aniline to highly ordered polyaniline–sulfate salt with a nanofiber morphology and its use as electrode materials in symmetric supercapacitors. J. Appl. Polym. Sci. 120, 780-788 (2011).
CrossRef
- S. Palaniappan, B. H. Narayana, Composition and spectral studies of polyaniline salts. Polym. Adv. Technol. 5, 225-230 (1994).
CrossRef
- H. D. Nguyen, T. H. Nguyen, N. V. Hoang, N. N. Le, T. N. N. Nguyen, D. T. Doan, M. C. Dang, pH sensitivity of emeraldine salt polyaniline and poly(vinyl butyral) blend. Adv. Nat. Sci. Nanosci. Nanotechnol. 5, 045001 (2014).
CrossRef
- Shao, W.W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline, Materials. 5, 1811-1825 (2012).
CrossRef
- N. K. Verma, Effect of Zinc Oxide Nano Particle Concentration in the Polyaniline-Zinc Oxide Nanocomposite on the Dielectric Property. Mat. Sci. Res. India. 11, 146-152 (2014).
CrossRef
- G. Ramesh, S. Palaniappan, A novel process of alcohol promoted polymerization of aniline to form a nanofibrous, fluorescent and highly crystalline polyaniline salt. New. J. Chem. 39, 8545-8551 (2015).
CrossRef
- D. Sudha Kumar, M. Iwamoto, Investigations on chiroptical behaviour of optically active polyaniline synthesized from naturally occurring amino acids. Polymer J. 45, 160-165 (2013).
CrossRef
Views: 3,702
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Ramesh Gottam
, Ramesh Gottam Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal