Introduction
Hydroxyapatite (HAp) Ca10 (PO4)6(OH)2 is one of the widely used replacement bioceramics in terms of bone and tooth substituent due to its high corrosion resistance, better compressive strength, porosity, low density and low weight.1 Porous morphology of HAp and β-TCP(Tri calcium phosphate) are attractive for bone regeneration and good growth property. HAp is the best alternative for bone and tooth replacement because of its similarity in terms of chemical structure, crystallography, morphology and Ca/P ratio of 1.67 with that of humans,2, 3,4,5 HAp is also is also used for non-medical application in terms of packing column in chromatography, gas sensors, and catalysts.6
The nature of the precursor, additives, method of synthesis used determine the composition, crystal structure, size, shape and morphology of the resulting Hydroxyapatite. HAp. In today’s context, there is need to prepare multifunctional HAp and hence several synthetic approaches like sol gel, solvothermal, wet chemical precipitation, are carried out with variation in precursors, experimental parameters, additives and modifiers. The precursors of Calcium carbonate HAp exists in hexagonal crystal structure and their natural resources being corals, sea urchins, oyster shell, and egg shell. Among them oyster shells (Crassostreao virginica) are abundant in the coast of Bay of Bengal in India.7 The present study aimed in tapping the natural sea waste and convert into valuable bio material and further modifying the material with the components of potent medicinal plant extract (stem and leaf) of neem (Azadirachta indica).
The leaves, twigs, and seeds of plant of Neem (Azadirachta indica) have been widely used for medicinal purposes due to their immense anti-bacterial, anti-fungal, anti-viral, anti-diabetic properties. More importantly they play a significant role as dentalcare products as they contain several anti-microbial compounds belonging to diterpenoids family namely margolone, margolonone and isomargolonone. They are reported to prevent dental plaque formation mostly caused by streptococcus mutans bacteria and candida albicans fungi. Also, the demineralization of enamel is found to be reversed by treating with Hydroxyapatite. The present study is also aimed at combining the unique Osteo-dental property of Hydroxyapatite and anti microbial activity of neem plant extract.8 In the study,the chemical constitution, micro structure and the anti-microbial activity of the synthesized Hydroxyapatite after modification with neem extract was explored.
Results
FT-IR Spectrum of Crassostreao Virginica Seashell After Calcination:
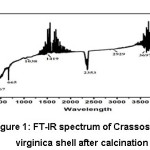
The purity of the sea waste (sea shell) in terms of calcium carbonate was evaluated using FT IR spectrum. In the FT-IR spectrum of calcined Crassostrea virginica seashell powder used as the precursor in the present study, a peak was observed at 1038cm-1 due to symmetric stretching C-O (ν1) of calcium carbonate which correlated with the report [6]. The peak was observed at 1419cm-1(ν3) which is due to doubly degenerate asymmetric stretching which is prominent peak of C-O in calcium carbonate,7,8,9,10 The peak observed at 467cm-1 due to stretching vibration of Ca-O bond was also identified in the calcined seashell. A peak was observed at 3697cm-1 due to O-H stretching which is more prominent in oyster shell.9,11
Figure 1: FT-IR spectrum of Crassostreao virginica shell after calcination
FT-IR Spectrum of Hydroxyapatite Obtained from Crassostreao Virginica seashell
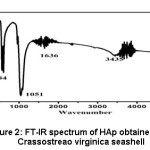
In the FT-IR spectrum of hydroxyapatite as obtained from calcined Crassostreao virginica shell as shown in the figure 2, there was a peak at 463cm-1 due to Ca-O bond, which is correlated with the report.9,11 A peak was observed at 584cm-1 due to asymmetric bending vibration of PO43-, which is well correlated with the reports.10,12 The peaks observed at 1051cm-1 due to asymmetric stretching vibration of P-O bond and the broad band in the region 3435cm-1, 1636cm-1 due to stretching vibrations of -OH group in hydroxyapatite also agreed with the available reports.11,13
Figure 2: FT-IR spectrum of HAp obtained from Crassostreao virginica seashell
FT-IR Spectrum of Hydroxyapatite Modified with Azadirchata indica (leaf, stem) using Crassostreao virginica seashell as precursor
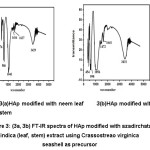
The FT-IR spectra of Hydroxyapatite modified with azadirchata indica extract obtained from Crassostreao virginica shell as shown in the figure 3. showed a peak at 476cm-1 (with neem leaf) and 454cm-1 (with neem stem)due to Ca-O bond.9,11 The peaks observed at 586cm-1 (with neem leaf) and 590cm-1 (with neem stem)are due to asymmetric bending vibration of PO43-, and well correlated with the reports.10,12 The peak observed at 1043cm-1 (neem leaf) and 1056cm-1 (neem stem) due to asymmetric stretching vibration of P-O bond and the broad band in the region 3429cm-1, 1637cm-1 (neem leaf) and 3431cm-1, 1641cm-1 (neem stem) due to stretching vibrations of -OH group in hydroxyapatite were well correlated with the available reports.11,13
Figure 3: (3a, 3b) FT-IR Spectra of HAp Modified with Azadirchata indica (leaf, stem) extract using Crassostreao virginica seashell as precursor
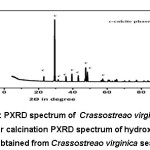
Figure 4: PXRD spectrum of Crassostreao virginica seashell after calcination
PXRD Spectrum of Hydroxyapatite Obtained from Crassostreao virginica Seashell
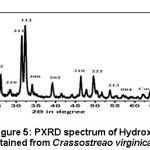
The PXRD spectrum of HAp obtained from calcined Crassostreao virginica seashell as given in the figure 5, clearly indicated the presence of HAp in the hexagonal phase (JCPDS 09-0432 and 01-09-1243). The presence of single phase of calcium oxide was also observed in HAp may be due to incomplete conversion of CaCO3 to Hydroxyapatite at 6500C.9,11 The average crystallite size of hydroxyapatite was found to be 38.42nm. The formation of hexagonal phased HAp in the nano scale is phenomenal since it resembles that of human tooth enamel.15,16,17
Figure 5: PXRD spectrum of Hydroxyapatite obtained from Crassostreao virginica seashell
PXRD Spectrum of Hydroxyapatite Obtained from Crassostreao Virginica Seashell Modified with Azadirchata Indica Extract (leaf):
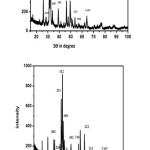
The PXRD spectrum of Hydroxyapatite HAp modified with azadirchata indica leaf extract as given in the figure 6, indicated the presence of HAp in the hexagonal phase (JCPDS 09-0432 and 01-09-1243). The presence of single phase of calcium oxide (2θ = 64.180) was observed in HAp which may be due to incomplete conversion of CaCO3 to Hydroxyapatite at 6500C.9 The average crystallite size of Hydroxyapatite was found to be 49.53nm and 52.48nm after modification with neam leaf and neem stem respectively. This clearly indicated that that Hydroxyapatite obtained from Crassostreao virginica shell in the nanoscale and hexagonal phase is like that of human tooth enamel.16,17,18
Figure 6: (6a, 6b). PXRD Spectrum of Hydroxyapatite Modified with Azadirchata Indica Extract (leaf, stem) Obtained from Crassostreao Virginica Shell
Scanning Electron Microscopy (SEM)
SEM Image of Hydroxyapatite Obtained from Crassostrea Virginica Shell
The surface morphology of HAp powder obtained from calcined Crassostreao virginica shell figure 7. The near spherical shape on the surface indicating formation of hydroxyapatite. The agglomeration was found to be less which may be due the presence of other elements assisting in the formation of well defined morphology of the sample. Porous morphology of HAp on the surface is attractive for bone regeneration, enhanced biodegradation and good growth property. The presence of small cluster of tidy grains indicates formation of the calcium oxide phase on the surface of hydroxyapatite. This observation also correlates with the report.16,17
Figure 7: SEM Image of Hydroxyapatite Obtained from Crassostreao virginica Shell
SEM Image of Hydroxyapatite Modified with Azadirchata Indica Extract (leaf, stem) as Medium Using Crassostreao Virginica Seashell
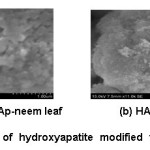
The surface morphology of hydroxyapatite modified with azadirachata indica [12(a) with leaf extract and 12(b) with stem extract] are shown in the figure 8. The HAp modified with azadirachata indica obtained from Crassostreao virginica shell observed to have near spherical shape. The HAp modified with stem extracts having higher crystallinity with large agglomeration than that of leaf extract. The flower like shape obtained from modification HAp with stem extract. The presence of small aggregates indicates formation of the calcium oxide phase on the surface of HAp. This observation also correlates with the report.17,18
Figure 8: SEM image of hydroxyapatite modified with azadirchata indica extract [neem leaf (a) and neem stem (b)] using Crassostreao virginica seashell
Energy Dispersive X Ray Analysis (EDAX)
The results of EDAX analysis done for synthesis of hydroxyapatite obtained from Crassostreao virginica seashell and modification of HAp with azadirchata indica extract as medium using Crassostreao virginica seashell, Anadara granosa seashell, synthetic calcium carbonate are tabulated in the Table (1). The Ca/P ratio was intended for 1.67 at the time of preparation, The Ca/P ratio was found to be near for modification HAp with azadirchata indica extract. This Ca/P ratio is like that of HAp present in bone and tooth of human. So that seashells are suitable precursor for synthesis of HAp. This is correlated with the report.18,19
Table 1: Comparison of weight percentage of Ca and P and their ratio of HAp and modified HAp samples with Azadirchata indica extract using Crassostreao virginica seashell as a precursor
|
Name of the sample
|
Weight percentage of calcium
|
Weight percentage of phosphorous
|
Ca/P ratio of modified HAp
|
Ca/P ratio of without modification of HAp
|
|
HAp-Crassostreao virginica seashell
|
27.56
|
16.94
|
–
|
1.63
|
|
HAp-Crassostreao virginica seashell–neem leaf
|
32.90
|
12.90
|
2.55
|
–
|
|
HAp- Crassostreao virginica virginica seashell-neem stem
|
23.51
|
13.97
|
1.68
|
–
|
Antimicrobial Activity of HAp and HAp Modified with Azadirchata Indica Extract
Nancy.F et al.,19,20 reported that common dental infections arise from streptococcus mutans bacteria and candida albicans fungi. Hence in the present study toxicity of HAp towards those and bacteria was focused. Antibacterial and antifungal studies of hydroxyapatite obtained from seashell and modified HAps are tabulated in the table (2) and table (3).
Antibacterial Activity
Antibacterial activity of hydroxyapatite obtained from seashell and modification of HAp are tabulated in table 2. There was 50 percent toxicity to streptococcus mutans cells at the concentration level of 500 µg in both the HAp samples with respect to the positive control of tetracycline (500 µg). The enhanced anti-bacterial activity of hydroxyapatite modified with azadirchata indica than that of HAp obtained from seashell may be attributed to the presence of other anti-microbial elements in neem extract and seashell viz:- zinc, Magnesium added from the precursor of seashell. This correlated with the report H. S. Ragab.20,21
Table 2: Antibacterial Activity of Hydroxyapatite and Modified Hydroxyapatite with Azadirchata Indica Extract
|
Name of the sample
|
modification
|
antibacterial activity
|
concentration
|
zone inhibition
|
|
sample
|
positive control
|
|
HAp-Crassostreao virginica seashell
|
Without
|
Name of the bacteria :Streptococcus mutans
|
2000
|
22
|
36
|
|
HAp-Crassostreao virginica seashell (leaf)
|
With
|
Name of the bacteria :Streptococcus mutans
|
2000
|
20
|
38
|
|
HAp-Crassostreao virginica seashell (stem)
|
With
|
Name of the bacteria :Streptococcus mutans
|
2000
|
20
|
39
|
Antifungal Activity
The results of antifungal activity on the azadirachata indica HAp and HAp modified with azadirchata indica extract are tabulated as table 3. The antifungal activities are not encouraging. It was observed that there was 50 percent toxicity to candida albicans cells at the concentration level of 1500 µg compared to the control of ketaconazole (positive control 500 µg). The antifungal activity of HAp modified with azadirchata indica extract was found to be nil even at higher concentration of 2000 μg/ml. The toxicity of neem leaf aqueous extract was reported at a concentration level of 2000μg.21,22 The antimicrobial constituents of neem undergo decomposition at the calcination temperature of 6500C. The residual organic components of the neem after decomposition would have played a role in the solubility of the sample in the solvent taken for fungal study.23
Table 3: Antifungal Activity of Hydroxyapatite and Modified Hydroxyapatite with Azadirchata Indica Extract
|
Name of the sample
|
modification
|
antifungal activity
|
concentration
|
zone inhibition
|
|
sample
|
positive control
|
|
HAp-Crassostreao virginica seashell
|
without
|
Name of the bacteria : Candida albicans
|
2000
|
10
|
27
|
|
HAp- Crassostreao virginica seashell (leaf)
|
With
|
Name of the bacteria : Candida albicans
|
2000
|
–
|
27
|
|
HAp- Crassostreao virginica seashell (stem)
|
With
|
Name of the bacteria : Candida albicans
|
2000
|
–
|
27
|
Discussion
The biowaste of seashell (Crassostreao virginica shell) was identified as a rich source of calcium carbonate. Aragonite and calcite form of CaCO3 was converted into highly stable calcite phase at lower calcination temperature of 7090C. Such potential bio waste into a nanoscale (38.42nm) Hydroxyapatite and modified HAp [49.53nm (neem leaf) and 52.48nm (neem stem)] using azadirachta indica extract by wet chemical precipitation method at lower calcination temperature of 6500C. The average crystallite size was found to be increased during modification. The microstructure, surface morphology and elemental composition (Ca/P ratio) of the as synthesized HAp and modified HAp samples matched very well with the HAp found in human bone. Porous morphology of HAp on the surface is attractive for bone regeneration, enhanced biodegradation and good growth property. The antibacterial (Streptococcus mutans) and antifungal (Candida albicans) activity of the as synthesized HAp was enhanced when seashell was used as the precursor for Ca. Also, the in situ modification of HAp carried out with the leaf and stem extract of Azadirachata indica influenced more on the surface morphology but very less on the antimicrobial (bacterial and fungal) activity. Hence the present study ultimately provided an energy efficient, economically cheaper method of converting sea waste into bio mineral with enhanced bio-active and bio compatible properties for wide ranging applications.
Materials and Methods
Materials
The chemicals used for the synthesis of hydroxyapatite were seashell (Crassostreao virginica seashell) powder which was used as natural precursor, azadirachta indica extract (leaf and stem), phosphoric acid (Molychem), hydrochloric acid, ammonium hydroxide. These crassostero virginica species are not frequently found in India but particularly available in the seashores of Gulf of Mannar namely, Rameshwaram which were taken up for the present study. They were cleaned with hot water, dil.HCl and bleaching powder and dried.
Wet Precipitation Method
Crassostreao virginica seashell was calcined at 7090C for 4hrs in a muffle furnace. The Aragonite and calcite form of oyster shell converted into calcite form of calcium carbonate during calcination. The calcite form of calcium carbonate obtained from oyster shell was converted into calcium chloride using hydrochloric acid.
7090C
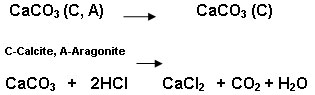
Calcium chloride (0.5M) obtained from the above process was treated with 0.3M phosphoric acid and ammonium hydroxide and keeping the pH at 10 and the reaction proceed up to one hour at 400C so that Ca/P=1.67.
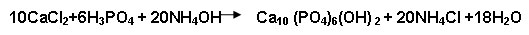
The obtained precipitate was filtered, dried at 900c for up to 24hrs. Then the precipitate was calcined at 6500C for about 6hrs in muffle furnace. The similar method was used for modification of HAp using azadirachta indica extract as medium. The assynthesized hydroxyapatite was ground for further characterization.
Scheme of In Situ Modification of Hydroxyapatite Using Neem Stem and Leaf Extract [Azadirachata Indica]
Analysis of the Samples by Instrumental Methods
The presence of functional groups was identified from FT-IR spectra using FT-IR (SHIMADZU) spectrometer. The spectra were collected in the range frequency range of 400-4000cm-1 and recorded against the background of KBr. The nature of crystallinity, crystal size, of the HAp samples were determined from the Powder X-ray Diffraction patterns (PXRD). They were recorded using CuKα radiation with the diffraction angle between 20° and 80°. PXRD analysis was performed using a PANalytical model Xpert pro instrument. The average crystallite size ‘D’ of the sample was calculated using the Scherrer equation, D=Kλ/βcosӨ, D- crystallite size of the sample, K-Scherrer constant (0.9), β-full width half maximum, ϴ- diffraction angle. The surface morphology and composition of the seashell samples were studied from the Scanning Microscopic (SEM) images. It was carried out using F E IQUANTA FEG 200. The relative percentage of elements at the surface of the images was determined from the Energy dispersive X-Ray analysis data (EDAX).
Anti Microbial (Anti Bacterial and Anti Fungal Activity Of Hydroxyapatites
The synthesized samples were also screened for antibacterial and antifungal activity against human pathogens such as streptococuus mutans and candida albicans using agar well diffusion method.
Samples for the study were prepared by dissolving 10mg of the hydroxyapatites in 500 µl of Ethanol + 500 µl of Acetone + 500 µl of Isopropanol. Nutrient Agar (NA) plates were inoculated with test microorganisms and were evenly spread out. Each well was loaded with 500,1000,1500,2000 µl of corresponding concentration of sample and 10 mg of Tetracycline dissolved in 1 ml of DMSO was used as a Positive control for antibacterial activity. Ketaconazole (100μg/well) was used as a positive control for antifungal activity. The plates were incubated for 24h at 37°C. The development of inhibition zone around the well was measured (diameter) and recorded.
Acknowledgments
The authors would like to thank Ethiraj College for Women, Chennai, India for their support and encouragement.
Funding Source
The author(s) declare(s) that the funding is done by author only.
References
- Hasan Mahmud Md, KaziAbdus Salam, Gafur M. A, AshequlAlam Rana, Rakibul Qadir Md, Masum Shah Md, Mithun Sarker, Mohammad Mainul Karim. Chemical Characteristics of Hydroxyapatite from Oyster Shell by Thermo-Chemical Process. International Journal of Innovative Research in Science Engineering and Technology. 2015; 7:5040-5047.
- Mehdi ebrahimi, Michael G.botelho, sergey V.Dorozhkin. Biphasic calcium phosphate bioceramic (HA/TCP): Concept, physico chemical properties and the impact of standardisation of study protocols in biomaterials research. Material science and engineering. 2017;71:1293-1312.
CrossRef
- Mythili Prakasam, Janis Locs , Kristine Salma-Ancane , Dagnija Loca, Alain Largeteau, Liga Berzina-Cimdina. Fabrication. Properties and Applications of Dense Hydroxyapatite: A Review. Journal of Functionalised Biomaterial. 2015;6:1099-1140.
CrossRef
- Linbo Dou,1 Yancong Zhang , 1 and Hanwen Sun 2, Advances in Synthesis and Functional Modification of Nanohydroxyapatite, Journal of Nanomaterials Volume 2018, Article ID 3106214, 7 pages.
- Humair A. Siddiqui 1,2, Kim L. Pickering 1 and Michael R. A Review on the Use of Hydroxyapatite Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes Mucalo 3,* Materials. 2018;11(10):1813;1-32.
- Sawittree Rujitanapanich, Piton Kumpapan, PanthongWanjanoi. Synthesis of hydroxyapatite from oyster shell via precipitation. 11th Eco-Energy and Material Science and Engineering. 2014; 56: 112-117.
CrossRef
- Hadeel Alobeedallah, Jeffrey L.Ellis, Ramin Rohanizadeh, Hanscoster, fabrica dehghani. Preparation of nanostructered hydroxyapatite in organic solvent in clinical application. Materials. 2011;25:12-19.
- Dhyani, Sadhna Tripathi. Quercetin-a potential compound of azadirachta indicaa.juss.(neem) leaves exhibiting activity against wood decaying fungi and termitesswati 38 Wood Preservation Discipline Forest Products Division, Forest Research Institute Dehradun, India
- Enamulhoque Md, Muhammed shehryar, khandakan, Nurul Islam Md. Processing and characterization of cockle shell calcium carbonate bioceramic for potencial applicationin bone tissue enginnering. Journal of Material Science and Engineering. 2013; 2: 1-6.
- Nurul Islam Kh, Zuki Bin Abu Bakar Md, EaqubAli Md, MohdZobir Bin Hussein, Mustapha M. Noordin, Loqman M.Y, Gous Miah, Hanif Wahid, UdaHashim. A novel method for the synthesis of calcium carbonates (aragonite) nanoparticles from cockle shells. Powder Technology. 2013;235:70-75.
CrossRef
- Dadkhah M, Salavati-Niasari M, Mir N. Synthesis and Characterization of Nano-Size CaCO3 via Thermal Treatment and Solid State Method. Journal of Nanostructures. 2012;2:153-158.
- Migule galvin ruiz, Mario enriquez Rodriguez-garica. Characterization of calcium carbonate, calcium oxide and calcium hydroxide as starting point to the improvement of lime for. Journal of materials in civil engineering. 2009;4: 1-20.
CrossRef
- Adrian paz, Dainelys geradarama monica, copez, jesus E. Comparative study of hydroxyapatite nanoparticle synthesized by different routes. Biomaterial and nanostructures group. 2012;35:1-8.
CrossRef
- Sudhaparimala S*, Usha.R. Quality (Nanoscale) assessments of Calcium carbonate present in shells of Anadara granosa, and Crassostreao virginica marine species located in the coastal part of South India. Advances in Natural and Applied science. 2017;11(9):205-211.
- Arunseshan chandrasekar, Suresh sagadevan, Arivuoli Dakshna moorthy. Synthesis and characterization of nano hydroxyapatite using the wet chemical precipitation technique. Journal of Material Science and Engineering. 2013; 32;1639-1645.
- Piergiorgio Gentile, caroline J.wilcock, Miller A, Robert Moorehead, paul V.Hattron. Process optimization of control the physic- chemical characteristics of biomimetic nanoscale hydroxyapatite prepared using wet chemical precipitation. Materials. 2015;8:2297-2310 .
CrossRef
- Mei-sheng Xia, Zhi-tong Yao, Liu-qinGe, Tao Chen, Hai-yan Li. A potential bio-filler: The substitution effect of furfural modified clam shell for carbonate calcium in Poly propylene. Journal of Composite Materials 2014;0(0):1–10.
- Nurhayati, Muhdarina, Amilia Linggawati, Sofiaanita, Tengku Ariful Amri. Preparation and characterization of calcium oxide Heterogeneous Catalyst Derived from Anadara granosa shell for Biodiesel synthesis. Environment and Renewable Energy. 2015;2016:1-8.
- Nurfatirah Nordin, Zainab Hamzah, Othman Hashim, Farizul Hafiz Kasim, Rozaini Abdullah. Effect of temperature in calcination process of seashells. Malaysian Journal of Analytical Sciences. 2015;19(1):65-70.
- Nancy F, Crum-cianflone. Bacterial, Fungal, Parasitic, Viral Myositis. Clinical Microbiology reviews. 2008; 21(3):474-494.
- Ragab H. S, Ibrahim F. A, Abdallah F, Attieh A, Al-Ghamdi, Farid El-Tantawy, Neyara Radwan, Yakup hanoglu. F. Synthesis and In Vitro Antibacterial Properties of Hydroxyapatite Nanoparticles. IOSR journal of pharmacy and biological science. 2014;7:77-85.
CrossRef
- Syed sibte asghar abidi, qasim murtaza. Synthesis and characterization of nano hydroxyapatite powder using wet chemical precipitation. U.P.B. Sci. Bull.,series . 2013;75:3-12.
- Himanshu khandewal, Satya prakesh. Synthesis and characterization of hydroxyapatite powder by egg shell. Journal of Minerals and Materials Characterization and Engineering. 2016;4:119-126.
CrossRef
Views: 1,178
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal