Improved Photoresponse in Association with a Synthesized Dielectric Material for Quantum Dots Solar Cells
Subhasis Roy* , Argha Dey, Bhaskar Chandra Das
, Argha Dey, Bhaskar Chandra Das
Department of Chemical Engineering, University of Calcutta India.
Corresponding Author Email: srchemengg@caluniv.ac.in
DOI : http://dx.doi.org/10.13005/msri/160305
Article Publishing History
Article Received on : 25-Nov-2019
Article Accepted on : 16-Dec-2019
Article Published : 17 Dec 2019
Plagiarism Check: Yes
Reviewed by: Ramalingam  Second Review by: Vijendra Singh Solanki
Second Review by: Vijendra Singh Solanki  Final Approval by: Dai-Viet N. Vo
Final Approval by: Dai-Viet N. Vo
Article Metrics
ABSTRACT:
A worldwide investigation is being carried out for improving the photoconversion efficiency of solar cells. Among all solar cells, quantum dots solar cell (QDSC) has proven as the best potential for photocurrent generator. The major focus of this research work is comparing the performance of QD based solar cells with and without the addition of synthesized dielectric nanomaterials for reducing recombination problems and higher the exciton generation. The selection of dielectric nanomaterial was carried out based on their good field-effect passivation, screened columbic attraction, enactment as a back reflector, and recombination inhibitor in solar cell. According to the above-mentioned factors lanthanum doped lead titanate Pb0.85La0.15TiO3 (PLT15) is a promising material for this research work. For improving the performance of QD based solar cells, the PLT15 paired mesoporous TiO2 electron transport layer (ETL) film was deposited onto fluorine-doped tin oxide (FTO) coated glass substrate using doctor blading technique followed by annealing the QD deposition onto the coated glass substrate was carried out via dipping of the glass into the QD solution for overnight. The QD used in this research work were namely – PbI3. Finally, the performance study was carried out which indicates that the introduction of dielectric material into the QDSC has proven to be as innovative and as well as efficient for improving the photocurrent conversion efficiency.
KEYWORDS:
Dielectric; Nanoparticles;Quantum dots; Thin film
Copy the following to cite this article:
Roy S, Dey A, Das B.C. Improved Photoresponse in Association with a Synthesized Dielectric Material for Quantum Dots Solar Cells.Mat. Sci. Res. India; 16 (3).
|
Copy the following to cite this URL:
Roy S, Dey A, Das B.C. Improved Photoresponse in Association with a Synthesized Dielectric Material for Quantum Dots Solar Cells.Mat. Sci. Res. India; 16 (3). Available from: http://bit.ly/36J362g
|
Introduction
In recent years the peaks of crisis in energy, change in climate, less availability of fossil-based fuel and concerning environmental pollution are trending towards the highest limiting range. As a result, to nurture the environment by protecting sustainable and renewable energy resources has become an essential trend worldwide [1–4]. The story of success lies within the development in an efficient manner of conversion of energy and storage devices. The present day’s low-carbon economy is dependent on the minimize utilization of fossil fuels and enhance the utilization of photovoltaic conversion [5–8]. Among the other kind of photovoltaic cells, quantum dots solar cell (QDSC) have owed lots of attention due to their simple technology associated with fabrication, high efficiency achieved through power conversion, and scalable materials [9–12]. Quantum dots solar cell (QDSC) is also capable of converting energy from light to electricity based upon the sensitization of metal oxide semiconductors having a wide-bandgap [13, 14].
Recently the attention has been gained by third-generation photovoltaics due to the fabrication of silicon nanocrystals embedded into the dielectric matrix [15]. Dielectric material coating offers high chemical passivation by means of dropping the surface density, which results recombination for photo–excited charge movers [15-17]. Also using dielectric material offers passivation by means of high field-effect through the highly dense amount of intrinsically fixed charges [18–20]. In one of our previous research work, we report synthesis, characterization and performance study of quantum dot material CdS embedded with Pb0.85La0.15TiO3 (PLT15) nano dielectric composite materials in the form of thin film for solar cell application. The performance study indicated improved photoconversion efficiency after the addition of dielectric material into the quantum dot-matrix [21].
In this present research work, the dielectric nanomaterial was introduced into the electron transport layer (ETL-TiO2) matrix as well as separated layer on ETL to achieve an improve photoconversion efficiency. This dielectric material lanthanum doped lead titanate (PLT15) was synthesized according to the earlier mentioned procedure [21]. This time it was introduced on the top of TiO2 matrix as well as mixed with TiO2 paste. Post annealing of the PLT15 doped TiO2 onto FTO glass substrate it was then dipped into the extracted QD solution (PbI3) overnight to attain proper deposition. The fabricated solar cell underwent for checking electrical performance analysis in the presence of simulated solar irradiation which was found that introducing dielectric materials enhance the photovoltaic performance of the solar cells.
Experimental
Synthesis of TiO2 nanoparticles
The synthesis was carried out according to the mentioned procedure [21]. This synthesis procedure involved the dropwise addition of titanium isopropoxide (97% Sigma–Aldrich) within 0.1 M nitric acid solution under rigorous stirring at room temperature. The volumetric amount during the mixing of titanium iso–propoxide and 0.1 M nitric acid kept as 5ml and 10 ml respectively. The dropwise addition of titanium iso–propoxide within 0.1 M nitric acid formed instant white precipitation. Further at 80°C temperature, 1000 rpm rotational speed the slurry underwent for 8h heating as well as stirring to achieve peptized agglomerates. Additional water was added into the mixture to balance the concentration upto~5 wt%. Then 12 hour heating within the temperature range of 230–250°C was employed on the solution for the growth of 10–25 nm particles. This heating was carried out with the help of a Teflon-lined stainless steel autoclave. The re-dispersion of the particles was done with the help of titanium ultrasonic horn (120 W, 15 pulses for 15 min). Finally, the colloidal suspension underwent concentration for 1 hour at 70°C into an oven.
Synthesis of Pb0.85La0.15TiO3 (PLT15) nanoparticles
This synthesis was also carried according to the mentioned procedure [21]. The synthesis involved lead acetate tri-hydrate [Pb(CH3COO)2 3H2O] (99.99% Sigma–Aldrich), lanthanum nitrate hexahydrate (La(NO3)3, 6H2O) (99.999% Sigma–Aldrich), and titanium butoxide [Ti(OC4H9)4] (97% Sigma–Aldrich) as raw materials. Under continuous stirring in presence of warm acetic acid Lead acetate tri-hydrate and lanthanum nitrate were allowed for dissolving. Glacial acetic acid was also taken into another three-neck flask for the dissolving of stoichiometric titanium butoxide such that the molar ratio of titanium butoxide and glacial acetic acid can be maintained as (1:2). Finally, the chelated sol was prepared through the dropwise addition of the mixed solution containing lead acetate tri-hydrate and lanthanum nitrate (through continuous stirring for 15–20 min) to prepare PLT15 precursor sol.
Fabrication of QDSC solar Cell
The buffer layer of TiO2 originated from Titanium isopropoxide was deposited onto fluorine-doped tin oxide (FTO) glass substrate by means of spin coating technique. Before coating, the fluorine-doped tin oxide (FTO) glass substrate (15 Ω/cm, Pilkington) was cleaned by detergent solution, water, and ethanol respectively in an ultrasonic bath. The layer of TiO2 underwent heating at 450 oC in a furnace for organic removal. Then the nano layer of TiO2 along with synthesized dielectric material PLT15 was coated onto the substrate by employing a doctor’s blade technique. Again the coated FTO substrate underwent heating. Further, the TiO2 coated FTO substrate was immersed overnight into the mixture of extracted dye solution for sensitization. The very next day the glass substrate was taken out of the solution. The coated glass was subjected to atmosphere for drying. The layer of spiro-OMeTAD (Sigma-Aldrich, 99%) was given onto the substrate using spin coater instrument. Further it was then allowed for atmospheric drying. Conductive silver paste was used for the preparation of top and bottom electrodes.
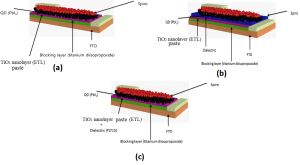
Figure 1: Schematic of the fabricated QD based solar cells (a) without dielectric materials addition (b) separated dielectric layer on ETL (L-TiO2) (c) mixed with electron transport layer (ETL-TiO2) matrix
Characterization with the extracted and synthesized material
The performance analysis of the fabricated solar cell in terms of photovoltaic current–voltage (PV-IV) characteristics was analyzed using a solar simulator connected with simulated standard sunlight (100 mW/cm2, AM 1.5G) (model: 2400 High Current Source Meter, Keithley) (Compact 150W Solar Simulator, Zolix Instruments, China).
Results and Discussion
Electrical characterization
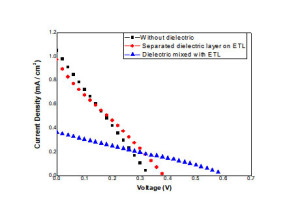
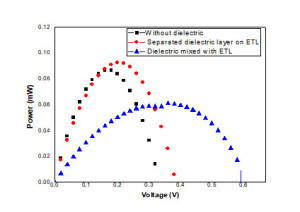
The electrical characterization of the fabricated solar cells in the presence and absence of PLT15 was characterized under simulated solar irradiation (1.5 suns). The input power for efficiency calculations is 1 kW/m2 or 100 mW/cm2. Figure 2 represents the current density vs voltage plot in the presence and absence of PLT15 respectively. From the obtained data point the corresponding open-circuit voltage (VOC), short circuit current (ISC), Fill factor (FF) and conversion efficiency have been obtained. The following table 1 represents the obtained data. Figure 3 shows the power –voltage (J–V) characteristics of solar cell. From the obtained data it is clear that dielectric mixed with electron transport layer (ETL-TiO2) matrix as improved the efficiency of the fabricated solar cell.
Figure. 2. The current-voltage (J–V) characteristics of solar cell
Figure. 3. The power –voltage (J–V) characteristics of solar cell
|
|
Cell parameters
|
|
Voc (Volt)
|
Jsc (mA/ cm2)
|
Fill Factor
|
Efficiency (%)
|
|
Cell parameters in absence of dielectric materails
|
0.33
|
1.05
|
0.24
|
3.32
|
|
Separated dielectric layer on ETL
|
0.38
|
0.8
|
0.3
|
3.64
|
|
Dielectric mixed with electron transport layer (ETL-TiO2) matrix
|
0.61
|
0.39
|
0.55
|
4.53
|
Table 1. Photovoltaic parameters of the solar cell
Conclusion
Mostly quantum dots solar cells undergo from low power conversion efficiency owing to the recombination of inorganic semiconductors. This novel hybrid dielectric based quantum dots solar cells produced stable and improved Isc, Voc and sufficient efficiency due to the addition of dielectric components (PLT15). Through this research work, the fabrication of dielectric inspired solar cells was carried out using synthesized material PLT15. The fabrication was carried out in the presence and absence of dielectric material. Electrical measurement was performed to check the suitability as light sensitizing material for both cases. The photovoltaic performance of the fabricated solar cell was carried out under simulated solar light (100mW/cm2 AM 1.5G). The efficiency showed that the addition of PLT15 in the solar cell matrix with the electron transport layer has increased the photoconversion efficiency due to several reasons which include lower of the surface recombination, better charge collection properties, and increase in the factor of the separation of the charge carriers by introducing photon. Additional investigation will focus on the effect of defects and impurities that interchange the electron-hole pair creation, a better understanding of transport properties, minority carrier lifetime that would finally lead to commercial large scale application.
Acknowledgments
This research work was supported by Science and Engineering Research Board (SERB) grants funded by the Department of Science and Technology (DST) Central, Government of India through Teachers Associateship for Research Excellence (TAR/2018/000195).
Funding Source
This research work was funded by the Science and Engineering Research Board vide Teachers Associateship for Research Excellence (TAR/2018/000195).
References
- M. Z. Jacobson, Energy Environ. Sci., 2, 148–173 (2009)
- S. A. Moyez, S. Roy, Sol. Energy Mater. Sol. Cells 185, 145 (2018).
- S. A. Moyez, S. Roy, J. Nanopart. Res. 20, 5 (2018).
- A. Moyez, A. Dhar, P. Sarkar, H. S. Jung, S. Roy, Reviews in Advanced Sciences and Engineering, American Scientific Publishers 5(1), 51 (2016).
- J. Burschka, N. Pellet, S.J. Moon, R. Humphry-Baker, P. Gao, M.K. Nazeeruddin, M. Gr€atzel, Nature 499, 316 (2013)
- M.C. Beard, J.M. Luther, A.J. Nozik, Nat. Nanotechnol. 9, 951 (2014).
- S. Kazim, M.K. Nazeeruddin, M. Gr€atzel, S. Ahmad, Angew. Chem. Int. Ed. 53 (2014) 2812.
- S. Pan, Z. Yang, P. Chen, J. Deng, H. Li, H.S. Peng, Angew. Chem. Int. Ed. 53, 6110 (2014).
- A. Hagfeldt, G. Boschloo, L.C. Sun, L. Kloo, H. Pettersson, Chem. Rev. 110 (2010) 6595e6663.
- U. Bach, T. Daeneke, Angew. Chem. Int. Ed. 51 (2012) 10451e10452.
- J.H. Wu, Y.M. Xiao, Q.W. Tang, G.T. Yue, J.M. Lin, M.L. Huang, Y.F. Huang, L.Q. Fan, Z. Lan, S. Yin, T. Sato, Adv. Mater. 24 (2012) 1884e1888.
- Y.M. Xiao, G.Y. Han, Y.P. Li, M.Y. Li, Y.Z. Chang, J. Mater. Chem. A 2 (2014) 3452e3460
- B. O’regan, M. Grätzel, Nature 353, 737–740 (1991).
- T.S. Feild, D.W. Lee, N.M. Holbrook, Plant Physiol. 127, 566–574 (2001).
- Manjceevan, A., Bandara, J., Sol. Energy Mater. Sol. Cells 147, 157 (2016).
- Wu, P.-J., Wang, Y.-C., Chen, I.-C., 2013. Nano Res. Lett. 8, 457 (2013).
- A. Mesquita, M. I. B. Bernardi, C. Godart, P. S. Pizani, A. Michalowicz, V. R. Mastelaro, Ceramics International 38, 5879 (2012)
- K. Zhao, R. Munir, B. Yan, Y. Yang, T. Kim, A. Amassian, J. Mater. Chem. A 3, 20554 (2015).
- Lare, C.V., Lenzmann, F., Verschuuren, M.A., Polman, A., Nano Lett. 15, 4846 (2015).
- S. A. Moyez, S. Roy, Materialstoday: proceedings 4(14), 12657 (2017).
- A. Dey, P. Karan, A. Sengupta, S. A. Moyez, P. Sarkar, S. Roy, Solar Energy 158, 83 (2017).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Argha Dey, Bhaskar Chandra Das
, Argha Dey, Bhaskar Chandra Das

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal