Effect of Temperature on the Performance Factors and Durability of Proton Exchange Membrane of Hydrogen Fuel Cell: A Narrative Review
Introduction
In the face of global warming, carbon pollution, fossil fuel decline hydrogen fuel cell is an exciting new platform to cope up with both fuel and environmental issues.1,2 In the modern world, there is a huge demand for hydrogen fuel in the industries. Hydrogen gas can be produced from fossil fuel (coal, gas, petroleum etc.) or renewable energy sources (solar, wind, geothermal energy, biogas, biomass, etc.).3,4 This hydrogen gas can be a universal fuel that can be produced using all existing fuel sources and can be stored for any time of future use. In a fuel cell, electricity is produced with a chemical reaction between hydrogen and oxygen where hydrogen gives an electron by oxidation reaction to the anode and becomes an electron less hydrogen ion called proton.4-6 This proton passes through the proton exchange membrane and reacts with oxygen by a reduction reaction with the formation of water and heat. In the external circuit, the electron flow gives the output current load. We can get electricity from an electrochemical reaction with zero emission of harmful chemicals and gases. That’s why the fuel cell technology is considered to be sustainable and emission-free fuel solution for future demand.7-10 The fuel cell has a wide range of applications. Because it operates with less start-up-time, zero knocking, no carbon emission and modern electricity production technology. It can be a source of energy for vehicles, industries, electricity demand, and even household chores. A fuel cell can meet MW level power demand in the grid. The power of the fuel cell may remain side by side of the grid. When the grid power becomes low then the fuel cell power can be a good alternative source of power with kW level.
In the electrochemical reaction in a fuel cell, there is a generation of heat along with the electricity and water.4-8,11 This heat can be used in the water boiling system with 90% efficiency. Fuel cells are being used in vehicles as a mobile battery.12-14 Without polluting the environment, electricity can be produced along with heat and water from hydrogen and oxygen gas fed into the PEMFC. In this reaction process, there are a lot of parameters to be considered to make the reaction process continue for a long duration without interruption. The different factors affecting the architecture and the performance of a fuel cell must be explored in a precise manner in the way of progress.12-19 Among all the parameters, the temperature of a fuel cell is a significant performance changing factor.7-9, 14 In this review paper we will focus on the effects of temperature on the performance and durability of hydrogen fuel cell and more specifically, a Proton Exchange Membrane Fuel Cell (PEMFC).
Mechanism of Hydrogen Fuel Cell
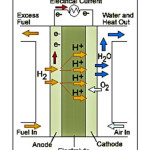
The main theme of a PEMFC is to produce electricity from the electrochemical reaction between hydrogen and oxygen reactant. 1,2 In this reaction electricity, heat, and water are produced. This electrochemical reaction happens with two electrodes separate with a proton exchange membrane. In the anode side, a hydrogen oxidation reaction occurs and in the cathode side, an oxygen reduction reaction occurs.8 For uniform reaction over the surface of the electrode, there is a gas diffusion layer. This gas diffusion layer will drive the gas all over the electrode to control the optimum performance of the reaction. Oxidation and reduction reactions are a slow process without any catalyst. For better and continuous operation, we need to introduce catalyst in the electrode. In the anode side, hydrogen gas is changed into hydrogen atom (proton, H+) and the electron goes through the outer circuit.14 Now only proton can pass through the proton exchange membrane. Hydrogen or oxygen gas is not permitted to pass through the proton exchange membrane. On the cathode side, oxygen gas comes from the air and passes through the gas diffusion layer, and with the help of the oxygen reduction reaction electricity, water, and heat are produced. 14,17 Figure 1 shows the mechanism of the PEMFC.
The full reaction is mentioned below. 17-21, 24,25
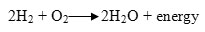
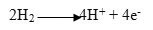
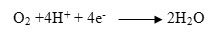
Anode Half Reaction:
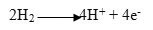
Cathode Half Reaction:
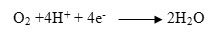
Among fuel cells, the proton exchange membrane fuel cell (PEMFC) gather much attention because of some unique criteria such as low thermal condition of operation, greater efficiency, shorter in size.10 The proton exchange membrane has hydrophilic and hydrophobic characteristics at the same time. Table 1 shows the types of PEMFC and their features. The design of the membrane remains durable for the hydrophobic part of the membrane, on the other hand, the hydrophilic part helps the membrane to remain hydrated for the optimum operation of the PEMFC.22,24-30 Water in the hydrated membrane helps proton to dissociate from the sulfonic acid part of the membrane material and the hydrogen ion (Proton) transfer from the anode side to the cathode side through the membrane. The input hydrogen gas must be pure up to 99.99%.
Table 1: Types of Proton Exchange Membrane Fuel Cell.
|
PEMFC Type
|
Operating Temperature
|
Features
|
|
High Temperature-PEMFC
|
Above 100℃
|
I. Durable in high CO condition
II. Well heat releasing system
III. Humidification not required
|
|
Low Temperature-PEMFC
|
60℃ to 80℃
|
I. Gas permeability resistance is high.
II. Cost effective technology
|
Factors Affecting the Performance of Hydrogen Fuel Cell
The performance of a PEMFC can be affected by many reasons. The load current, temperature, relative humidity, membrane thickness, membrane-active area, electrode active area, corrosion, purity, pressure, and concentration of hydrogen fuel, maintenance of water inside the cell, pressure in the electrode particularly on both side of the membrane etc. are the factors. 12-19, 22,28
Activation, ohmic and concentration losses make the fuel cell voltage less than before. 29 Humid condition and thermal condition are two significant factors for PEMFC operation. The change in the temperature of a fuel cell affects the electrochemical reaction, proton exchange, and water production. The bipolar plate has two sides.30,31 On one side there is a gas flow path and on the other side, external water flow is used for temperature maintenance in the fuel cell. Rising current density accelerates the reaction process. 17-19 Besides, the heat production in the fuel cell is proportional to the rate of the reaction process. In the outer part of the plate, the proton exchange membrane becomes dried because of the rising temperature. Additionally, the density of the electron flow rate becomes lower over time in the fuel cell. 19,28The density of the electron flow is higher when the supplied gas is enough. The current distribution is also an important factor and it is inversely proportional to the density of electron flow rate. The rising reactant flow rate creates a uniform current flow. Inappropriate thermal energy will decrease the performance of the proton exchange membrane. 12, 23-28
Effect of Temperature on PEMFC
Though several factors such as operating temperature, electrolytes used, humidity, catalyst, produced heat etc. have vital effects on the performance of Hydrogen Fuel Cell, in this paper, we will discuss the effects of temperature elaborately. The effects of temperature on different parameters are shown in Table 2 and discussed elaborately below:
Performance and Efficiency
Heat generates during the PEMFC operation. For better efficiency and consistence output there must be a cooling process either by air or fluid to get rid of the cell generated heat. 10The proton exchange membrane fuel cell shows better performance with the rise in temperature and pressure. Because the entropy change is small during the rise in temperature and pressure. A less chance of entropy indicates better and stable performance in a fuel cell. As the thermal energy is improved, the overall performance like current, current density, voltage, electricity production of a proton exchange membrane fuel cell improves. 24 It has been observed in another study that fuel cell performance increased when the temperature increased to 120℃. 32,33,37
Normally it is considered that the efficiency of the PEMFC is increased in terms of the increase in temperature. 22,36-40 A hydrated proton exchange membrane normally works in a range of temperatures below 100℃ and if we include some new elements in the PEMFC then the FC will operate in the temperature range higher than 100℃. 36,37 A new technology has been found for high-temperature fuel cells with a temperature range from 90℃ to 200℃. 40,44-46 At this high-temperature range from 90 to 200℃, the rate of proton exchange through the membrane becomes high and that’s why there is a rapid rise in reaction mechanism in anode and cathode. 40-46 The transfer of mass positively rises with the rise of the temperature.
Table 2: Effect of Temperature on PEMFC parameters
|
Parameters
|
Effect on the parameters
|
References
|
|
Performance and efficiency
|
Increases with the increase in temperature
|
22,32,36,37
|
|
Humidity
|
Optimum temperature maintains the required humidity
|
32,36,45-50
|
|
Power Production
|
Increases with the increase in temperature
|
18,32, 55,56
|
|
Voltage
|
Increases with the increase in temperature
|
47, 58-60
|
|
Leakage Current
|
Increases with the increase in temperature
|
61,62
|
|
Catalyst Tolerance
|
Increases with the increase in temperature
|
26,29,36, 40,66,67
|
|
Mass cross-over
|
Decreases with the increase in temperature
|
32,44-46
|
|
Durability
|
Decreases with the increase in temperature
|
40,48,71-76
|
Humidity
The proton exchange quality of the membrane depends on the humid condition of the membrane. 47-53 The presence of water in the membrane maintains the optimum humid condition. Adequate water is required for the membrane to be hydrated and the rest of the water needs to come out of the fuel cell for better performance. Otherwise, the extra water will create additional complications inside the fuel cell. At the same time, the temperature rise is one of the reasons for water loss in the membrane.22,32,36 When the temperature of hydrogen fuel remains high, the membrane becomes dehydrated. As a result, less amount of proton can pass through the anode to the cathode side which will reduce the electron flow and efficiency of the PEMFC. In high temperature and high humidity, membrane crossover of the hydrogen gas rises. It is one of the reasons for PEMFC decay. After the exchange of protons through the membrane with electrochemical reaction, water is produced. Excess water production will make the membrane wet by the diffusion process. 45Wet proton exchange membrane is very essential for proton exchange from anode to cathode. The electrochemical reaction would rapidly rise with the increase in temperature and would produce enough water. This water will make the membrane wet and again it will increase PEMFC efficiency. 45,53,54
Without optimum humid conditions in the membrane, the resistance of the membrane to hydrogen ion will rise. As a result, this rise in resistance will increase the temperature. To maintain the good condition of the fuel cell operation, an optimum humid condition should be maintained either by in-vitro or in-vivo water maintenance in the fuel cell. 47,48 The ion exchange is the main parameter to be observed during less humid conditions. Humidity controls the hydrogen and oxygen flow in both of the electrodes in the fuel cell. 48,49 Particularly the humidity in the cathode side creates a condition for operating a fuel cell in lower temperatures. The performance and durability of the membrane directly depend on the humid condition. If the humidity in the proton exchange membrane is up to 100%, it can lead to catalyst decay from the surface of the membrane. 47-50 Besides, in less humidity, the polymer electrolyte membrane turns into more brittle form and degrades faster, particularly the acid group of the membrane degrades, and the catalyst is washed away from the surface of the membrane. 32,36,47-50
The efficiency of power production
In a proton exchange membrane fuel cell, the density of power production rises by 16% for the operational temperature rise from 50℃ to 80℃. The power production efficiency of a PEMFC is increased with the increase of operational temperature.18, 32 The value of dissipated power is reduced and the initiated over-potential become less due to the rising temperature which results in increased power production efficiency.55-56
Voltage
According to the Nernst equation, the temperature is proportional to the output voltage.58 Higher temperature leads to faster kinetics and as a result, the voltage is also increased. This increase in voltage surpasses the voltage loss from the negative thermodynamic correlation between the open-circuit voltage and temperature.58-60 But in a study, the fuel cell was found to have worse performance due to unfortunate damage or hole in the proton exchange membrane at 70℃ operating temperature. 47 In this case, the voltage dropped in the fuel cell as the hydrogen gas passes through the membrane. So, if the voltage does not increase with the temperature increase, there might be damage in the proton exchange membrane.
Leakage Current
The membrane of PEMFC is regarded as hydrogen impermeable and electrically insulated. But leakage current still occurs within the fuel cell. It is often supposed to be around 0.01 A.cm-2 in PEM fuel cell simulation literature.61During the electrochemical reaction in the fuel cell, hydrogen gas, and electrons diffuse through the proton exchange membrane.62 For the diffusion process of hydrogen gas and electrons through the proton exchange membrane, a minute amount of current is produced. This current is known as leakage current. With the rise in temperature, the leakage current also increases. If the temperature rises from 50℃ to 80℃ then the leakage current density change will be 6-12 mA/cm2. The value of leakage current density will be constant with the constant value of temperature.
Catalyst Tolerance
The decay of the material of the components of the PEMFC is a very important factor in the performance of the PEMFC. The efficiency of catalyst decay over time depends on the hydrogen oxidation reaction, oxygen reduction reaction, high potential, and pH environment. Platinum catalyst plays a vital role in the performance of fuel cells. The oxygen reduction reaction in the cathode is a slow reaction process. To overcome the slowness, an effective catalyst can accelerate the oxygen reaction rate in the cathode which will improve the PEMFC efficiency rapidly. The energy conversion process in PEMFC is very efficient regarding the input hydrogen purity. 37 Otherwise less pure hydrogen will damage fuel cell components and operation. To produce low-cost hydrogen fuel with required purity is a goal to achieve to make fuel cells more feasible to use. If the hydrogen is not pure then carbon mono oxide will be produced and associates with the surface of the catalyst. That’s why the reaction hampered in the fuel cell.24-26 However, in the HT-PEMFC this carbon mono-oxide dissociation in the catalyst surface can be solved. At high temperature accelerate the reaction kinetic in a fuel cell. In high-temperature PEM become dehydrated. 26-29 As a result catalyst decay decreases, proton passes through membrane rapidly, electrochemical reaction accelerates, reaction remains active for a long time. High temperature affects the lifetime of the FC. Besides leaching of reaction acid should be maintained in HT- PEMFC. Water is produced in the chemical reaction at the anode side. This water production falls at the rise in the operational temperature range from 80℃ to 120℃. Rapid water production in the PEMFC will inundate the proton exchange membrane. A proton exchange membrane fuel cell has an efficiency of up to 60%. 26 The tolerance level of the catalyst to the contaminants in the membrane will rise significantly with temperature.40,65 When PEMFC operates at a temperature below 100℃, CO covers the catalyst layer. As a result, the electrochemical reaction process becomes slower. The CO accumulation in the catalyst surface reduces the 50% lifetime of the fuel cell. To ameliorate the bad effect of CO, a certain type of catalyst should be selected which has no reactive mechanism to this harmful gas.40,67
Mass Cross-Over and Concentration Over-Potential
Mass cross-over and concentration over-potential are also related to the temperature of the PEMFC. If the temperature rises, the mass cross-over falls and concentration over-potential rises. The current density becomes high.32 On the other hand, the activation over-potential remains static up to the 80℃. Then towards 100℃, the activation over-potential rises. It is considered that up to 80℃, the PEMFC efficiency remains in good condition. In anode and cathode, the activation over-potential decreases with the rise in temperature over 80℃. But in 120℃ the anode activation over-potential value is higher than that was between in the 80 to 100℃ range but in the cathode, the situation is opposite.44-46
Durability
Despite the immense evolution of the proton exchange membrane, the longevity is still a concern.76 The durability of the catalyst, electrode plate, gas diffusion layer, the gasket is directly related to the longevity of the proton exchange membrane.40-48 Electrochemical erosion, component erosion, and thermal effect are the leading factors for the longevity of the proton exchange membrane.71,76
The proton exchange membrane loses its water and becomes dehydrated with the rise of the temperature .40-46 As a result, the hydrogen gas crossing the dehydrated membrane will reach on the cathode side. Hydrogen in the cathode side will then damage the bipolar plate, catalyst, and gaskets. If it continues to operate at high temperatures, then over time the durability of the PEMFC will decrease. 28 A fluid dynamic model was proposed in research with an operating temperature range from 80℃ to 120℃ and pressure range at 200000Pa. In this model, a high-temperature fuel cell showed better performance with better current density at 80℃ rather than 120℃. The water production in the anode side was found to be better than that of in cathode side. A change in temperature from 120℃ to 80℃ was found to give smooth water production. But it can directly harm the fuel cell and the durability of the fuel cell will decrease.69 At low thermal conditions around 100℃, there is a minute amount of water accumulation in the surface of the proton exchange membrane.24 The sulfonate part of the Nafion membrane decays at high-temperature range around 200℃ which will permit the hydrogen gas to pass through the proton exchange membrane and reaches in cathode area.24,68
Effect of Input Hydrogen Gas Temperature
PEMFC has unique criteria such as low operating temperature & pressure, longevity, mobility and small size.1-8 The start-stop mechanism and output load stability are the major issues of PEMFC. Lack of enough input gas supply results in a rise in temperature in PEMFC. 44-46 Inadequate input gas supply creates a potential pressure in the anode. As a result, the temperature of PEMFC rises and creates decay in the membrane which makes a decay path. 24 In vehicle operation, the fuel cell temperature range is 80℃. But the stability of the PEMFC will be affected by this temperature. At changing operating temperatures such as 25℃,40℃,60℃, and 80℃ the fuel cell efficiency increased with the increasing temperature. The fuel cell efficiency increases when the start-stop round was in on mode and the stack efficiency decreased when the start-stop round was in off mode. But regardless the start-stop round was on or off with rising temperature the fuel cell can run with better efficiency.45
Effect of Internally Produced Heat
If the hydrogen and oxygen flow is not enough then there will be a rise in temperature in PEMFC. As a result, the overall performance of the fuel cell will fall. 24 Produced heat in the fuel cell during chemical reaction affects all the components (anode, cathode, gas diffusion layer, gasket, electron collector, membrane electrode assembly) and the operating condition (temperature, the humidity of the membrane, voltage, current) of the PEMFC.46-50,70-74
Lu et al in 2016 demonstrated that the Platinum palladium-based fuel cell reaction catalyst increases performance and output energy due to enhanced ion exchange during the reaction phase. If the resistance to ion exchange is lower, then the loss would be lower and the longevity would improve. Therefore, the extra heat production due to the resistance of the ion exchange would be lower.73
New Technology for Enduring High Temperature
In the rising market, there is a huge demand for polymer electrolyte membrane temperature range from 120℃ to 140℃. Because the water drainage system and heat processing are very easy in high-temperature PEMFC. 23 A new type of non-perfluorosulfonic acid membrane other than nafion or perfluorosulfonic acid is a good choice for cost-effective and durable in high-temperature condition with better performance. 23,75 It facilitates the use of high temperature. 45 By modifying different parameters and design constructions, high temperature can be used to have greater efficiency.
Conclusion
Hydrogen fuel cell is a promising source of renewable energy in upcoming days. But the system is still not economically feasible as the cost of construction is high, the relatively inexpensive catalyst is yet to be discovered, durability is not up to the mark, the cost of producing hydrogen gas is high, and so on. Fuel cell performance will vary depending on architectural design, component design, the chemical composition of components, atmospheric specifications, the parameters within the fuel cell, the best operating condition, the ability to generate energy. A continuous performance can be observed through a fuel cell test station setup. In this paper, we can conclude that temperature has significant effects on almost all ambient parameters and design components. So, considering all these effects, the optimum temperature should be used. As the higher temperature has been found to be beneficial in most cases, design variations are required to facilitate it. We have also discussed the design variations that can be useful for using high temperature. This review paper can lead to a proper combination and specification of the design components and factors to develop purpose-specific Hydrogen fuel cells.
Highlights
- Different factors have effects on the performance and durability of Hydrogen fuel cell.
- The effects of Temperature on the performance and durability of PEMFC have been discussed.
Acknowledgements
- M. Abdus Salam: Conceptualization, Writing, Review & Editing, Supervision
- Md Shehan Habib: Resources, Validation, Writing – Original Draft
- Paroma Arefin: Review & Editing
- Kawsar Ahmed: Writing – Original Draft
- Md. Sahab Uddin: Literature review, writing original draft
- Tareq Hossain: Literature review, writing original Draft
- Nasrin Papri: Literature review, writing original draft
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Staffell I, Scamman D, Velazquez Abad A, Balcombe P, Dodds PE, Ekins P, Shah N & Ward KR. The role of hydrogen and fuel cells in the global energy system. Energy & Environmental Science. Royal Society of Chemistry (RSC); 2019;12(2):463–91. Available from: http://dx.doi.org/10.1039/C8EE01157E
CrossRef
- Dodds PE, Ekins P. A portfolio of power trains for the UK: An energy systems analysis. International Journal of Hydrogen Energy [Internet]. Elsevier BV; 2014 Sep;39(26):13941–53. Available from: http://dx.doi.org/10.1016/j.ijhydene.2014.06.128
CrossRef
- Fuel cell today. 2013. The fuel cell industry review. Available from http://www.fuelcelltoday.com/.
- International Energy Agency Technology Roadmap: Hydrogen and Fuel Cells, Paris, 2015
- Hamrock SJ, Yandrasits MA. Proton Exchange Membranes for Fuel Cell Applications. Journal of Macromolecular Science, Part C: Polymer Reviews [Internet]. Informa UK Limited; 2006 Sep;46(3):219–44. Available from: http://dx.doi.org/10.1080/15583720600796474
CrossRef
- Cano ZP, Banham D, Ye S, Hintennach A, Lu J, Fowler M. Batteries and fuel cells for emerging electric vehicle markets. Nature Energy [Internet]. Springer Science and Business Media LLC; 2018 Apr;3(4):279–89. Available from: http://dx.doi.org/10.1038/s41560-018-0108-1
CrossRef
- Hosseini SE, & Wahid MA. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renewable and Sustainable Energy Reviews, 57, 850–866. doi:10.1016/j.rser.2015.12.112
CrossRef
- Salam MA, Ahmed K, Akter N, Hossain T, Abdullah B. A review of hydrogen production via biomass gasification and its prospect in Bangladesh. International Journal of Hydrogen Energy [Internet]. Elsevier BV; 2018 Aug;43(32):14944–73. Available from: http://dx.doi.org/10.1016/j.ijhydene.2018.06.043
CrossRef
- Bezmalinović D, Strahl S, Roda V, Husar A. Water transport study in a high temperature proton exchange membrane fuel cell stack. International Journal of Hydrogen Energy [Internet]. Elsevier BV; 2014 Jul;39(20):10627–40. Available from: http://dx.doi.org/10.1016/j.ijhydene.2014.04.186
CrossRef
- Budak Y, Özgirgin Yapıcı E, Devrim Y. Investigation of Working Temperature Effect on Micro-Cogeneration Application of Proton Exchange Membrane Fuel Cells. Hittite Journal of Science & Engineering [Internet]. Hitte Journal of Science and Engineering; 2018;5(Special). Available from: http://dx.doi.org/10.17350/hjse19030000116
CrossRef
- Vivek R and Muthukumar M. Performance Improvement of Proton Exchange Membrane Fuel Cell. Innovative Energy & Research, 2018, 07(02). doi:10.4172/2576-1463.1000203
CrossRef
- Aquino, A.K., & Heng, J.O. (2017). Current and Temperature Distributions in a PEM Fuel Cell.
CrossRef
- Zhao N, Chu Y, Xie Z, Eggen K, Girard F, Shi Z. Effects of Fuel Cell Operating Conditions on Proton Exchange Membrane Durability at Open‐Circuit Voltage. Fuel Cells [Internet]. Wiley; 2020 Apr;20(2):176–84. Available from: http://dx.doi.org/10.1002/FUCE.201900173
CrossRef
- Sengodan, S., Lan, R., Humphreys, J., Du, D., Xu, W., Wang, H., & Tao, S. (2018). Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renewable and Sustainable Energy Reviews, 82, 761–780. doi:10.1016/j.rser.2017.09.071
CrossRef
- Ching YC, Gunathilake TU, Ching KY, Chuah CH, Sandu V, Singh R, et al. Effects of high temperature and ultraviolet radiation on polymer composites. Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites [Internet]. Elsevier; 2019;407–26. Available from: http://dx.doi.org/10.1016/B978-0-08-102290-0.00018-0
CrossRef
- Al-Mufachi NA, Steinberger-Wilckens R. Influence of temperature and pressure on surface modified Pd-Cu alloy foils for hydrogen purification applications. Thin Solid Films [Internet]. Elsevier BV; 2018 Jan;646:83–91. Available from: http://dx.doi.org/10.1016/j.tsf.2017.11.032
CrossRef
- Dodds, P. E., Staffell, I., Hawkes, A. D., Li, F., Grünewald, P., McDowall, W., & Ekins, P. (2015). Hydrogen and fuel cell technologies for heating: A review. International Journal of Hydrogen Energy, 40(5), 2065–2083. doi:10.1016/j.ijhydene.2014.11.059
CrossRef
- Khan SS, Shareef H, Wahyudie A, Khalid S, Sirjani R. Influences of ambient conditions on the performance of proton exchange membrane fuel cell using various models. Energy & Environment [Internet]. SAGE Publications; 2018 Oct 10;30(6):1087–110. Available from: http://dx.doi.org/10.1177/0958305×18802775
CrossRef
- Barbouche M, Ahmed Z, Charradi K, Chtourou R, Squadrito G. Effect of Temperature, Humidity and Gas Flow on PEM Fuel Cell Performances for Environmental Applications. Advances in Science, Technology & Innovation [Internet]. Springer International Publishing; 2018;1117–8. Available from: http://dx.doi.org/10.1007/978-3-319-70548-4_323
CrossRef
- Kim S, Hong I. Effects of humidity and temperature on a proton exchange membrane fuel cell (PEMFC) stack. Journal of Industrial and Engineering Chemistry [Internet]. Elsevier BV; 2008 May;14(3):357–64. Available from: http://dx.doi.org/10.1016/j.jiec.2008.01.007
CrossRef
- Ariza HE, Correcher A, Sánchez C, Navarro-Pérez Á, García E. Thermal and Electrical Parameter Identification of a Proton Exchange Membrane Fuel Cell Using Genetic Algorithm. MDPI AG; 2018 Jul 10; Available from: http://dx.doi.org/10.20944/preprints201807.0164.v1
CrossRef
- Jourdani M, Mounir H, Marjani AE. Latest Trends and Challenges In Proton Exchange Membrane Fuel Cell (PEMFC). The Open Fuels & Energy Science Journal [Internet]. Bentham Science Publishers Ltd.; 2017 Dec 20;10(1):96–105. Available from: http://dx.doi.org/10.2174/1876973X01710010096
CrossRef
- Escorihuela J, García-Bernabé A, Montero Á, Sahuquillo Ó, Giménez E, Compañ V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers [Internet]. MDPI AG; 2019 Apr 22;11(4):732. Available from: http://dx.doi.org/10.3390/polym11040732
CrossRef
- Gimba, I. D., Abdulkareem, A. S., Jimoh, A., & Afolabi, A. S. Theoretical Energy and Exergy Analyses of Proton Exchange Membrane Fuel Cell by Computer Simulation. Journal of Applied Chemistry, 2016, 1–15. doi:10.1155/2016/2684919
CrossRef
- Yonoff RE, Ochoa GV, Cardenas-Escorcia Y, Silva-Ortega JI, Meriño-Stand L. Research trends in proton exchange membrane fuel cells during 2008–2018: A bibliometric analysis. Heliyon [Internet]. Elsevier BV; 2019 May;5(5):e01724. Available from: http://dx.doi.org/10.1016/j.heliyon.2019.e01724
CrossRef
- Sun, H., Yu, M., Li, Z., & Almheiri, S. A Molecular Dynamic Simulation of Hydrated Proton Transfer in Perfluorosulfonate Ionomer Membranes (Nafion 117). Journal of Chemistry, 2015, 1–10. doi:10.1155/2015/169680
CrossRef
- Zeis R. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Beilstein Journal of Nanotechnology [Internet]. Beilstein Institut; 2015 Jan 7;6:68–83. Available from: http://dx.doi.org/10.3762/bjnano.6.8
CrossRef
- SALEH IMM, ALI R, ZHANG H. Simplified mathematical model of proton exchange membrane fuel cell based on horizon fuel cell stack. Journal of Modern Power Systems and Clean Energy [Internet]. Springer Science and Business Media LLC; 2016 Apr 15;4(4):668–79. Available from: http://dx.doi.org/10.1007/s40565-016-0196-5
CrossRef
- Amphlett JC, Mann RF, Peppley BA, Roberge PR, Rodrigues A. A model predicting transient responses of proton exchange membrane fuel cells. Journal of Power Sources [Internet]. Elsevier BV; 1996 Jul;61(1-2):183–8. Available from: http://dx.doi.org/10.1016/S0378-7753(96)02360-9
CrossRef
- Bose S, Kuila T, Nguyen TXH, Kim NH, Lau K, Lee JH. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Progress in Polymer Science [Internet]. Elsevier BV; 2011 Jun;36(6):813–43. Available from: http://dx.doi.org/10.1016/j.progpolymsci.2011.01.003
CrossRef
- Budak Y, Özgirgin Yapıcı E, Devrim Y. Investigation Of Working Temperature Effect On Micro-Cogeneration Application Of Proton Exchange Membrane Fuel Cells. Hittite Journal of Science & Engineering [Internet]. Hitte Journal of Science and Engineering; 2018;5(Special). Available from: http://dx.doi.org/ .17350/hjse19030000116
CrossRef
- Esfeh HK, Hamid MKA. Temperature Effect on Proton Exchange Membrane Fuel Cell Performance Part II: Parametric Study. Energy Procedia [Internet]. Elsevier BV; 2014;61:2617–20. Available from: http://dx.doi.org/10.1016/j.egypro.2014.12.261
CrossRef
- Hamrock SJ, Yandrasits MA. Proton Exchange Membranes for Fuel Cell Applications. Journal of Macromolecular Science, Part C: Polymer Reviews [Internet]. Informa UK Limited; 2006 Sep;46(3):219–44. Available from: http://dx.doi.org/10.1080/15583720600796474
CrossRef
- Oono Y, Fukuda T, Sounai A, Hori M. Influence of operating temperature on cell performance and endurance of high temperature proton exchange membrane fuel cells. Journal of Power Sources [Internet]. Elsevier BV; 2010 Feb;195(4):1007–14. Available from: http://dx.doi.org/10.1016/j.jpowsour.2009.08.097
CrossRef
- Jin Zhang, David Aili, Shanfu Lu, Qingfeng Li, and San Ping Jiang, “Advancement toward Polymer Electrolyte Membrane Fuel Cells at Elevated Temperatures,” Research, vol. 2020, Article ID 9089405, 15 pages, 2020. https://doi.org/10.34133/2020/9089405.
CrossRef
- Jourdani M, Mounir H, El Marjani A. Compilation of factors affecting durability of Proton Exchange Membrane Fuel Cell (PEMFC). 2014 International Renewable and Sustainable Energy Conference (IRSEC) [Internet]. IEEE; 2014 Oct; Available from: http://dx.doi.org/10.1109/IRSEC.2014.7059906
CrossRef
- Colmati F et al.Production of Hydrogen and their Use in Proton Exchange Membrane Fuel Cells, Advances In Hydrogen Generation Technologies, Murat Eyvaz, IntechOpen, DOI: 10.5772/intechopen.76663.2018. Available from: https://www.intechopen.com/books/advances-in-hydrogen-generation-technologies/production-of-hydrogen-and-their-use-in-proton-exchange-membrane-fuel-cells
- Chandan A, Hattenberger M, El-kharouf A, Du S, Dhir A, Self V, et al. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC) – A review. Journal of Power Sources [Internet]. Elsevier BV; 2013 Jun;231:264–78. Available from: http://dx.doi.org/10.1016/j.jpowsour.2012.11.126
CrossRef
- Liu, Y., Lehnert, W., Janßen, H., Samsun, R. C., & Stolten, D. (2016). A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. Journal of Power Sources, 311, 91–102. doi:10.1016/j.jpowsour.2016.02.033
CrossRef
- Zhang J, Zhang H, Wu J, Zhang J. The Effects of Temperature on PEM Fuel Cell Kinetics and Performance. Pem Fuel Cell Testing and Diagnosis [Internet]. Elsevier; 2013;121–41. Available from: http://dx.doi.org/10.1016/B978-0-444-53688-4.00004-8
CrossRef
- Liu Y, Gao J, Pei P, Yao S, Wang F, Qin H. Effects of dynamic changes in inlet temperature on proton exchange membrane fuel cell. Journal of Renewable and Sustainable Energy [Internet]. AIP Publishing; 2019 Jul;11(4):044302. Available from: http://dx.doi.org/10.1063/1.5050300
CrossRef
- Legree M, Sabatier J, Mauvy F, Awad A salam, Faessel M, et al. Autonomous Hydrogen Production for Proton Exchange Membrane Fuel Cells PEMFC. Journal of Energy and Power Technology [Internet]. LIDSEN Publishing Inc; 2020 Apr 28;2(2):1–18. Available from: http://dx.doi.org/10.21926/JEPT.2002004
CrossRef
- Liang H, Su H, Pollet BG, Pasupathi S. Development of membrane electrode assembly for 41high temperature proton exchange membrane fuel cell by catalyst coating membrane method. Journal of Power Sources [Internet]. Elsevier BV; 2015 Aug;288:121–7. Available from: http://dx.doi.org/10.1016/j.jpowsour.2015.04.123
CrossRef
- Pistono AO, Rice CA. Automotive Subzero Cold-Start Quasi-Adiabatic Proton Exchange Membrane Fuel Cell Fixture: Design and Validation. Molecules [Internet]. MDPI AG; 2020 Mar 19;25(6):1410. Available from: http://dx.doi.org/10.3390/MOLECULES25061410
CrossRef
- Sirliyani, Devianto H, Nurdin I. Effect of hydrogen temperature and current load on the performance of proton exchange membrane fuel cell under start-stop operation. Proceedings of the Joint International Conference on Electric Vehicular Technology and Industrial, Mechanical, Electrical and Chemical Engineering (ICEVT & IMECE) [Internet]. IEEE; 2015 Nov; Available from: http://dx.doi.org/10.1109/ICEVTIMECE.2015.7496712
CrossRef
- Jeppesen C, Araya SS, Sahlin SL, Thomas S, Andreasen SJ, Kær SK. Fault detection and isolation of high temperature proton exchange membrane fuel cell stack under the influence of degradation. Journal of Power Sources [Internet]. Elsevier BV; 2017 Aug;359:37–47. Available from: http://dx.doi.org/10.1016/j.jpowsour.2017.05.021
CrossRef
- Ferraris A, Messana A, Airale AG, Sisca L, de Carvalho Pinheiro H, Zevola F, et al. Nafion® Tubing Humidification System for Polymer Electrolyte Membrane Fuel Cells. Energies [Internet]. MDPI AG; 2019 May 10;12(9):1773. Available from: http://dx.doi.org/10.3390/en12091773
CrossRef
- Saleh M.M.; Okajima, T.; Hayase, M.; Kitamura, F.; Ohsaka, T. Exploring the effects of symmetrical and asymmetrical relative humidity on the performance of H2/air PEM fuel cell at different temperatures. J. Power Sources 2007, 164, 503–509.
CrossRef
- Bi W, Sun Q, Deng Y, Fuller TF. The effect of humidity and oxygen partial pressure on degradation of Pt/C catalyst in PEM fuel cell. Electrochimica Acta [Internet]. Elsevier BV; 2009 Feb;54(6):1826–33. Available from: http://dx.doi.org/10.1016/j.electacta.2008.10.008
CrossRef
- Chen C, Fuller TF. The effect of humidity on the degradation of Nafion® membrane. Polymer Degradation and Stability [Internet]. Elsevier BV; 2009 Sep;94(9):1436–47. Available from: http://dx.doi.org/10.1016/j.polymdegradstab.2009.05.016
CrossRef
- Obayopo SO, Bello-Ochende T, Meyer JP. Thermodynamic Optimization of PEM Fuel Cell Stack Gas Channel for Optimal Thermal Performance. 2010 14th International Heat Transfer Conference, Volume 5 [Internet]. ASMEDC; 2010 Jan 1; Available from: http://dx.doi.org/10.1115/IHTC14-22233
CrossRef
- Wan Z, Chang H, Shu S, Wang Y, Tang H. A Review on Cold Start of Proton Exchange Membrane Fuel Cells. Energies [Internet]. MDPI AG; 2014 May 13;7(5):3179–203. Available from: http://dx.doi.org/10.3390/en7053179
CrossRef
- Nam JH, Kaviany M. Effective diffusivity and water-saturation distribution in single- and two-layer PEMFC diffusion medium. International Journal of Heat and Mass Transfer [Internet]. Elsevier BV; 2003 Nov;46(24):4595–611. Available from: http://dx.doi.org/10.1016/S0017-9310(03)00305-3
CrossRef
- Liu Y, Bai S, Wei P, Pei P, Yao S, Sun H. Numerical and Experimental Investigation of the Asymmetric Humidification and Dynamic Temperature in Proton Exchange Membrane Fuel Cell. Fuel Cells [Internet]. Wiley; 2020 Jan 28;20(1):48–59. Available from: http://dx.doi.org/10.1002/FUCE.201900140
CrossRef
- Khan, S. S., Shareef, H., Wahyudie, A., Khalid, S., & Sirjani, R. (2019). Influences of ambient conditions on the performance of proton exchange membrane fuel cell using various models. Energy & Environment, 30(6), 1087–1110. https://doi.org/10.1177/0958305X18802775
CrossRef
- Khan SS, Shareef H, Mutlag AH. Dynamic temperature model for proton exchange membrane fuel cell using online variations in load current and ambient temperature. International Journal of Green Energy [Internet]. Informa UK Limited; 2019 Jan 26;16(5):361–70. Available from: http://dx.doi.org/10.1080/15435075.2018.1564141
CrossRef
- Pérez-Page M, Pérez-Herranz V. Effect of the Operation and Humidification Temperatures on the Performance of a PEM Fuel Cell Stack. ECS Transactions [Internet]. The Electrochemical Society; 2019 Dec 17;25(1):733–45. Available from: http://dx.doi.org/10.1149/1.3210625
CrossRef
- Li C, Liu Y, Xu B, Ma Z. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes [Internet]. MDPI AG; 2019 Jul 3;7(7):419. Available from: http://dx.doi.org/10.3390/pr7070419
CrossRef
- Khotseng L. Fuel Cell Thermodynamics , IntechOpen. 2019. DOI: 10.5772/intechopen.90141. Available from: https://www.intechopen.com/online-first/fuel-cell-thermodynamics
CrossRef
- Fuel Cell Operating Conditions. 2017 . Available from https://www.fuelcellstore.com/blog-section/fuel-cell-operating-conditions
CrossRef
- Rezaei Niya SM, Phillips R, Hoorfar M. Estimation of Leakage Current in Proton Exchange Membrane Fuel Cells. ECS Transactions [Internet]. The Electrochemical Society; 2014 Sep 19;61(23):33–8. Available from: http://dx.doi.org/10.1149/06123.0033ecst
CrossRef
- Hahn R, Wagner S, Krumbholz S, Reichl H. Optimization of efficiency and energy density of passive micro fuel cells and galvanic hydrogen generators. 2008 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS [Internet]. IEEE; 2008 Apr; Available from: http://dx.doi.org/10.1109/DTIP.2008.4752947
CrossRef
- Bi W, Fuller TF. Temperature Effects on PEM Fuel Cells Pt∕C Catalyst Degradation. Journal of The Electrochemical Society [Internet]. The Electrochemical Society; 2008;155(2):B215. Available from: http://dx.doi.org/10.1149/1.2819680
CrossRef
- Holton OT, Stevenson JW. The Role of Platinum in Proton Exchange Membrane Fuel Cells. Platinum Metals Review [Internet]. Johnson Matthey; 2013 Oct 1;57(4):259–71. Available from: http://dx.doi.org/10.1595/147106713X671222
CrossRef
- Miao Z, Yu H, Song W, Hao L, Shao Z, Shen Q, et al. Characteristics of proton exchange membrane fuel cells cold start with silica in cathode catalyst layers. International Journal of Hydrogen Energy [Internet]. Elsevier BV; 2010 Jun;35(11):5552–7. Available from: http://dx.doi.org/10.1016/j.ijhydene.2010.03.045
CrossRef
- Sun X, Simonsen SC, Norby T, Chatzitakis A. Composite Membranes for High Temperature PEM Fuel Cells and Electrolysers: A Critical Review. Membranes (Basel). 2019 Jul 11;9(7):83. doi: 10.3390/membranes9070083. PMID: 31336708; PMCID: PMC6680835.
CrossRef
- Iezzi R, Santos R, da Silva G, Paganin V, Ticianelli E. CO Tolerance and Stability of Proton Exchange Membrane Fuel Cells with Nafion® and Aquivion® Membranes and Mo‑Based Anode Electrocatalysts. Journal of the Brazilian Chemical Society [Internet]. Sociedade Brasileira de Quimica (SBQ); 2017; Available from: http://dx.doi.org/10.21577/0103-5053.20170230
CrossRef
- Wu, D., Peng, C., Yin, C. et al. Review of System Integration and Control of Proton Exchange Membrane Fuel Cells. Electrochem. Energ. Rev. (2020). https://doi.org/10.1007/s41918-020-00068-1
CrossRef
- Almeida RA, Rezende RVP, Cabral VF, Noriler D, Meier HF, Cardozo-Filho L, et al. The Effect Of System Temperature And Pressure On The Fluid-Dynamic Behavior Of The Supercritical Antisolvent Micronization Process: A Numerical Approach. Brazilian Journal of Chemical Engineering [Internet]. FapUNIFESP (SciELO); 2016 Mar;33(1):73–90. Available from: http://dx.doi.org/10.1590/0104-6632.20160331s20140016
CrossRef
- Simon Araya S, Grigoras IF, Zhou F, Andreasen SJ, Kær SK. Performance and endurance of a high temperature PEM fuel cell operated on methanol reformate. International Journal of Hydrogen Energy [Internet]. Elsevier BV; 2014 Oct;39(32):18343–50. Available from: http://dx.doi.org/10.1016/j.ijhydene.2014.09.007
CrossRef
- Shen, G., Liu, J., Wu, H.B. et al. Multi-functional anodes boost the transient power and durability of proton exchange membrane fuel cells. Nat Commun 11, 1191 (2020). https://doi.org/10.1038/s41467-020-14822-y
CrossRef
- Thomas A, Maranzana G, Didierjean S, Dillet J, Lottin O. Heat fluxes and electrodes temperature in a proton exchange membrane fuel cell. Mechanics & Industry [Internet]. EDP Sciences; 2012;13(4):255–60. Available from: http://dx.doi.org/10.1051/meca/2012021
CrossRef
- Lu Y, Du S, Steinberger-Wilckens R. Three-dimensional catalyst electrodes based on PtPd nanodendrites for oxygen reduction reaction in PEFC applications. Applied Catalysis B: Environmental [Internet]. Elsevier BV; 2016 Jun;187:108–14. Available from: http://dx.doi.org/10.1016/j.apcatb.2016.01.019
CrossRef
- Lux S, Baldauf-Sommerbauer G, Siebenhofer M. Hydrogenation of Inorganic Metal Carbonates: A Review on Its Potential for Carbon Dioxide Utilization and Emission Reduction. ChemSusChem. 2018 Oct 11;11(19):3357-3375. doi: 10.1002/cssc.201801356. Epub 2018 Aug 29. PMID: 30098275; PMCID: PMC6221144.
CrossRef
- Kraytsberg A, Ein-Eli Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy & Fuels [Internet]. American Chemical Society (ACS); 2014 Dec 10;28(12):7303–30. Available from: http://dx.doi.org/10.1021/ef501977k
CrossRef
- Shen, G., Liu, J., Wu, H.B. et al. Multi-functional anodes boost the transient power and durability of proton exchange membrane fuel cells. Nat Commun 11, 1191 (2020). https://doi.org/10.1038/s41467-020-14822-y
CrossRef
- Sauermoser M, Kizilova N, Pollet BG, Kjelstrup S. Flow Field Patterns for Proton Exchange Membrane Fuel Cells. Frontiers in Energy Research [Internet]. Frontiers Media SA; 2020 Feb 19;8. Available from: http://dx.doi.org/10.3389/FENRG.2020.00013
CrossRef
- Lee C-Y, Weng F-B, Kuo Y-W, Tsai C-H, Cheng Y-T, Cheng C-K, et al. In-Situ Measurement of High-Temperature Proton Exchange Membrane Fuel Cell Stack Using Flexible Five-in-One Micro-Sensor. Sensors [Internet]. MDPI AG; 2016 Oct 18;16(10):1731. Available from: http://dx.doi.org/10.3390/s16101731
CrossRef
- Li X, Ma H, Wang P, Liu Z, Peng J, Hu W, et al. Highly Conductive and Mechanically Stable Imidazole-Rich Cross-Linked Networks for High-Temperature Proton Exchange Membrane Fuel Cells. Chemistry of Materials [Internet]. American Chemical Society (ACS); 2020 Jan 2;32(3):1182–91. Available from: http://dx.doi.org/10.1021/ACS.CHEMMATER.9B04321
CrossRef
- Cao T-F, Mu Y-T, Ding J, Lin H, He Y-L, Tao W-Q. Modeling the temperature distribution and performance of a PEM fuel cell with thermal contact resistance. International Journal of Heat and Mass Transfer [Internet]. Elsevier BV; 2015 Aug;87:544–56. Available from: http://dx.doi.org/10.1016/j.ijheatmasstransfer.2015.04.010
CrossRef
Views: 20,572
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Paroma Arefin2
, Paroma Arefin2 , Kawsar Ahmed1, Md. Sahab Uddin1
, Kawsar Ahmed1, Md. Sahab Uddin1 , Tareq Hossain1 and Nasrin Papri1
, Tareq Hossain1 and Nasrin Papri1 
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal