Efficient Synthesis, Spectroscopic and Quantum Chemical Study of 2,3-Dihydrobenzofuran Labelled Two Novel Arylidene Indanones: A Comparative Theoretical Exploration
Rahul Ashok Shinde1,2 , Vishnu Ashok Adole2
, Vishnu Ashok Adole2 , Bapu Sonu Jagdale,1,2*
, Bapu Sonu Jagdale,1,2* , Thansing Bhavsing Pawar1
, Thansing Bhavsing Pawar1 , Bhatu Shivaji Desale2 and Rohit Shankar Shinde2
, Bhatu Shivaji Desale2 and Rohit Shankar Shinde2
1Department of Chemistry, Mahatma Gandhi Vidyamandir’s Loknete Vyankatrao Hiray Arts, Science and Commerce College Panchavati (Affiliated to SP Pune University, Pune), Nashik-422 003, India
2Department of Chemistry, Mahatma Gandhi Vidyamandir’s Arts, Science and Commerce College (Affiliated to Savitribai Phule Pune University, Pune), Manmad-423104
Corresponding Author Email: jagdalebs@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170207
Article Publishing History
Article Received on : 4 Jun 2020
Article Accepted on : 21 Jul 2020
Article Published : 22 Jul 2020
Plagiarism Check: Yes
Reviewed by: Mr.Sandip S. Pathade
Second Review by: Ayyappan Elangovan
Final Approval by: Vijai Anand
Article Metrics
ABSTRACT:
Indanone and 2,3-dihydrobenzofuran scaffolds are considered as special structures in therapeutic science and explicitly associated with various biologically potent compounds. In the present disclosure, we report the synthesis of two new 2,3-dihydrobenzofuran tethered arylidene indanones via an environmentally adequate and viable protocol. The two compounds revealed in this have been characterized well by analytical methods; proton magnetic resonance (PMR), carbon magnetic resonance (CMR). The Density Functional Theory (DFT) study has been presented for the spectroscopic, structural and quantum correlation between (E)-2-((2,3-dihydrobenzofuran-5-yl)methylene)-2,3-dihydro-1H-inden-1-one (DBDI) and (E)-7-((2,3-dihydrobenzofuran-5-yl)methylene)-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (DBTI). Optimized geometry, frontier molecular orbital, global reactivity descriptors, and thermodynamic parameters have been computed for DBDI and DBTI. DFT/B3LYP method using basis set 6-311++G (d,p) has been employed for the computational study. Mulliken atomic charges are established by using 6-311G (d,p) basis set. Besides, molecular electrostatic potential for DBDI and DBTI is also explored to locate the electrophilic and nucleophilic centres.
KEYWORDS:
DFT; HOMO-LUMO; Molecular Electrostatic Potential; 6-311++G(d,p); (E)-2-((2,3-dihydrobenzofuran-5-yl)methylene)-2,3-dihydro-1H-inden-1-one; (E)-7-((2,3-dihydrobenzofuran-5-yl)methylene)-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one
Copy the following to cite this article:
Shinde R. A, Adole V. A, Jagdale B. S, Pawar T. B, Desale B. S, Shinde R. S. Efficient Synthesis, Spectroscopic and Quantum Chemical Study of 2,3-Dihydrobenzofuran Labelled Two Novel Arylidene Indanones: A Comparative Theoretical Exploration. Mat. Sci. Res. India; 17(2).
|
Copy the following to cite this URL:
Shinde R. A, Adole V. A, Jagdale B. S, Pawar T. B, Desale B. S, Shinde R. S. Efficient Synthesis, Spectroscopic and Quantum Chemical Study of 2,3-Dihydrobenzofuran Labelled Two Novel Arylidene Indanones: A Comparative Theoretical Exploration. Mat. Sci. Res. India; 17(2). Available from: https://bit.ly/3jt8huf
|
Introduction
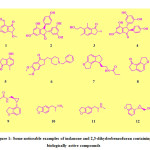
In the past few years, research on indanones and related compounds has been explored and they are found to exert an expansive range of biological properties.1-12 Importantly, they are the exceptionally valuable synthetic equivalents for the synthesis of wide variety of organic compounds having an excellent biologic profile.13-16 Many bioactive natural products are indanone based molecules. Some noticeable examples (Fig 1) 1-methoxy-6-methyl-3-oxo-2,3-dihydro-1H-indene-4-carbaldehyde (1), isopaucifloral F (2), 4-hydroxy-7-methyl-2,3-dihydro-1H-inden-1-one (3), Paucifloral F (4), Pterosin B (5). These noteworthy naturally derived compounds are known as powerful medicinal agents.17-22 Donepezil (6) is one of the most important indanone structures which have been proficiently used for the treatment of Alzheimer’s disease due to its acetyl cholinesterase inhibitor activity. On the other hand, some eye catching examples of drugs containing dihydrobenzofuran are tasimelteon (9), 1-(2,3-dihydrobenzofuran-5-yl)propan-2-amine (10), 1-(2,3-dihydrobenzofuran-5-yl)-N-methylpropan-2-amine (11) and ((1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropyl) methanol (12). The 2-arylidene indanone compounds have been investigated as potent agents for treatment of Alzheimer’s disease, breast cancer and leukaemia, as tubulin depolymerizing agents and many other significant pharmacological applications. 1, 23 A medicinally important structure which is being used as sleep inducing agent is Ramelteon (7). Ramelteon binds with MT-1 and MT-2 receptor. It contains a tricyclic synthetic molecule: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (8). The 2,3-dihydrobenzofuran natural products have pulled in expanding consideration because of their unusual structural highlights and the wide scope of biological activities.[24] The benzofuran and 2,3-disubstituted benzofuran scaffolds are present in countless natural products that exhibit incredible profile of biological activities, such as antimicrobial, antiviral, anti-inflammatory antioxidant, and anticancer activities.25-29 In recent times, these compounds have been investigated to have various biological activities.30-37 Henceforth benzofuran containing compounds may be used to design and generate new capacity therapeutic candidates having exceptional importance in the subject of the new drug research.38-40 The use of green chemistry principles has been great advantage for the environment.41-54
Theoretical calculations based on DFT have been successfully explored largely in past few years to determine various structural aspects of synthetically and pharmacologically vital organic motifs.55-58 DFT/B3LYP method using various basis set has been found to be very crucial for investigating structural, chemical, and spectroscopic properties of the molecules.59-63 Considering all mentioned properties and future scope of these molecules, we have designed 2,3-dihydrobenzofuran tethered two important synthetic compounds (scheme 1) and have been explored for the investigation of their structural, chemical, electronic, thermodynamic and quantum chemical parameters. To the best of our insights, this is preliminary report on synthesis, characterization and DFT investigation of title molecules.
Methodology
Materials and Methods
The chemicals with high purity were purchased from local distributor. The chemicals were used as received without any further purification. Melting point was determined in open capillary and uncorrected. 1H NMR and 13C NMR spectra were recorded with a Bruker using CDCl3 as solvent, FT-IR spectra were obtained with potassium bromide pellets. Reaction was monitored by thin-layer chromatography using aluminium sheets with silica gel 60 F254 (Merck).
Experimental Procedure for the Synthesis of DBDI and DBTI
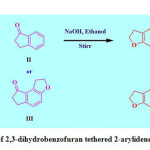
The synthesis of DBDI and DBTI is presented in Scheme 1. In a typical synthesis procedure, 2,3-dihydrobenzofuran-5-carbaldehyde (I, 10 mmol), 2,3-dihydro-1H-inden-1-one (II, 10 mmol) or 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (III, 8 mmol) was added to a 25 mL flat bottom flask. To this 2 mL 40% NaOH and 5 mL ethanol were added. Then this alkaline mixture was stirred on magnetic stirrer until the formation of desired products (checked by TLC (7:3; hexane: ethyl acetate). Reaction mass was transferred to a beaker containing ice cold water, then filtered, dried and recrystallized to offer fine yellow crystals of IV or V.
Computational Details
Density Functional Theory calculations were computed on an Intel (R) Core (TM) i5 computer using Gaussian-03 program package without any constraint on the geometry.64 The geometry of the title molecules was optimized by DFT/B3LYP method using 6-311++G (d,p) basis set. Optimized geometry was made using the Gauss View 4.1 molecular visualization program. To investigate the reactive sites of the title compound, the molecular electrostatic potential was computed using the same method. All the calculations were carried out for the optimized structure in the gas phase. Mulliken atomic charges were established by using 6-311G (d,p) basis set.
Results and Discussion
Spectral Analysis of DBDI and DBTI
The spectral images are given in Fig 2 (13 = 1H NMR spectrum of DBDI, 14 = 13C NMR spectrum of DBDI, 15 =1H NMR spectrum of DBTI, 16 = 13C NMR spectrum of DBTI).
a. (E)-2-((2,3-dihydrobenzofuran-5-yl)methylene)-2,3-dihydro-1H-inden-1-one (DBDI) : M.F. C18H14O2; Pale yellow crystals; M.P. 97 ºC; 1H NMR (500 MHz, Chloroform-d) δ – 7.90 (m, 1H), 7.64 (t, J = 2.1 Hz, 1H), 7.60 (td, J = 7.3, 1.2 Hz, 1H), 7.58 – 7.51 (m, 2H), 7.48 (dd, J = 8.3, 1.9 Hz, 1H), 7.47 – 7.38 (m, 1H), 6.86 (d, J = 8.3 Hz, 1H), 4.65 (t, J = 8.7 Hz, 2H), 3.99 (d, J = 2.1 Hz, 2H), 3.28 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, Chloroform-d) δ – 194.45, 161.81, 149.48, 138.33, 134.39, 134.29, 132.25, 131.76, 128.28, 128.19, 127.58, 127.54, 126.09, 124.28, 109.97, 71.95, 32.63, 29.40
b. (E)-7-((2,3-dihydrobenzofuran-5-yl)methylene)-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (DBTI) : M.F. C20H16O3; Yellow crystals; M.P. 147ºC; IR (KBr, cm-1) – 2916.37, 1643.35, 1564.27, 1490.97, 1427.32, 1328.95, 1280.73; 1H NMR (500 MHz, Chloroform-d) δ 7.56 (d, J = 2.2 Hz, 1H), 7.52 (s, 1H), 7.47 (dd, J = 8.3, 1.9 Hz, 1H), 7.28 (s, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.86 (d, J = 8.3 Hz, 1H), 4.67 (dt, J = 14.6, 8.8 Hz, 4H), 3.93 (d, J = 2.0 Hz, 2H), 3.56 (t, J = 8.9 Hz, 2H), 3.28 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, Chloroform-d) δ – 194.69, 161.73, 160.45, 141.39, 134.86, 133.83, 132.88, 132.20, 128.34, 128.13, 127.51, 125.03, 124.56, 115.13, 109.93, 72.44, 71.93, 32.23, 29.41, 28.54
Computational Study
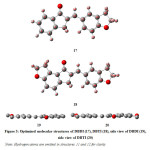
The optimized molecular structure of title molecules is depicted in Fig 3. Molecular structures 17 and 18 represent optimized geometrical structures for DBDI and DBTI respectively. The optimized molecular geometry provides good deal of information about the spatial orientation of various atoms in a molecule. From optimized molecular structures, it can be easily seen that both DBDI and DBTI possess C1 point group symmetry due to overall asymmetry of the molecules. Furthermore, it is evident that both the molecules contain non-planar dihydrofuran ring [Fig 3 (19 and 20)]. The non-planarity of dihydrofuran can be attributed to CH2 group (adjacent to oxygen atom) which is either above or below the plane. Due to this fact, both the molecules lack molecular plane (sh). It can also be seen that remaining skeleton of these molecules are in perfect planar position and therefore can have extended conjugation. This information is very much useful for the determination of various spectroscopic entities. The optimized geometrical parameters like bond lengths and bond angles of DBDI and DBTI are presented in Table 1A and 1B. The C9-O13 bond is 1.2195 Å in DBDI and C9- 1.2216 Å in DBTI. The olefinic C14-C15 bond is 1.3489 Å and 1.3491 Å in DBDI and DBTI molecules respectively. Other bond lengths are also in good agreement with the optimized structures. Bond angle data of both molecules are also in good agreement.
Table 1A: Optimized geometrical parameters of DBDI by DFT/ B3LYP with 6-311++G(d,p) basis set
|
Bond lengths (Å)
|
|
C1-C2
|
1.3955
|
C9-C14
|
1.4968
|
C20-C24
|
1.39
|
|
C1-C6
|
1.4016
|
C10-H11
|
1.0966
|
C22-C25
|
1.0828
|
|
C1-H7
|
1.0846
|
C10-H12
|
1.0966
|
C22-C24
|
1.396
|
|
C2-C3
|
1.392
|
C10-H14
|
1.5142
|
C22-C28
|
1.5123
|
|
C2-H8
|
1.0852
|
C14-C15
|
1.3489
|
C24-O34
|
1.3588
|
|
C3-C4
|
1.3967
|
C15-H16
|
1.0892
|
C28-H29
|
1.0949
|
|
C3- C10
|
1.5162
|
C15-C17
|
1.4554
|
C28-C30
|
1.5454
|
|
C4-C5
|
1.3949
|
C17-C18
|
1.4111
|
C28-H33
|
1.0919
|
|
C4-C9
|
1.485
|
C17-C19
|
1.4153
|
C30-H31
|
1.089
|
|
C5- C6
|
1.3908
|
C18-C20
|
1.3917
|
C30-H32
|
1.0935
|
|
C5- H26
|
1.0841
|
C18-H21
|
1.0803
|
C30-O34
|
1.4575
|
|
C6- H27
|
1.0841
|
C19-C22
|
1.3806
|
–
|
–
|
|
C9-O13
|
1.2195
|
C19-H23
|
1.0852
|
–
|
–
|
|
Bond angles (°)
|
|
C2-C1-C6
|
121.0843
|
C3-C10-H12
|
111.0202
|
C24-C20-H25
|
120.7525
|
|
C2-C1-H7
|
119.5183
|
C3-C10-C14
|
103.716
|
C19-C22-C24
|
119.7843
|
|
C6-C1-H7
|
119.3974
|
H11-C10-H12
|
106.8518
|
C29-C22-C28
|
132.2832
|
|
C1-C2-C3
|
118.7113
|
C11-C10-C14
|
112.1608
|
C24-C22-C28
|
107.8768
|
|
C1-C2-H8
|
120.2384
|
H12-C10-C14
|
112.1657
|
C20-C24-C22
|
121.8831
|
|
C3-C2-H8
|
121.0502
|
C9-C14-C10
|
108.9035
|
C20-C24-O34
|
124.6712
|
|
C2-C3-C4
|
119.9694
|
C9-C14-C15
|
119.6675
|
C22-C24-O34
|
113.443
|
|
C2-C3-C10
|
128.8339
|
C10-C14-C15
|
131.4291
|
C22-C28-H29
|
110.9107
|
|
C4-C3-C10
|
111.1967
|
C14-C15-H16
|
113.501
|
C22-C28-C30
|
101.3765
|
|
C3-C4-C5
|
121.5946
|
C14-C15-C17
|
131.9157
|
C22-C28-C33
|
113.4248
|
|
C3-C4-C9
|
109.8763
|
H16-C15-C17
|
114.5833
|
H29-C28-C30
|
111.6154
|
|
C5-C4-C9
|
128.529
|
C15-C17-C18
|
124.5419
|
H29-C28-H33
|
107.733
|
|
C4-C5-26
|
118.3789
|
C15-C17-C19
|
117.4689
|
C30-C28-H33
|
111.7831
|
|
C4-C5-C26
|
119.9287
|
C18-C17-C19
|
117.9891
|
C28-C30-C31
|
114.1679
|
|
C6-C5-C26
|
121.6924
|
C17-C18-C20
|
122.1142
|
C28-C30-H32
|
111.7492
|
|
C1-C6-C5
|
120.2615
|
C17-C18-H21
|
119.8067
|
C28-C30-O34
|
106.6458
|
|
C1-C6-C27
|
119.5862
|
C20-C18-H21
|
118.0781
|
H31-C30-H32
|
109.2705
|
|
C5-C6-C27
|
120.1523
|
C17-C19-C22
|
120.4148
|
H31-C30-O34
|
107.3237
|
|
C4-C9-O13
|
126.7014
|
C17-C19-H23
|
119.0694
|
H32-C30-O34
|
107.3357
|
|
C4-C9-H14
|
106.3076
|
C22-C19-H23
|
120.5157
|
C24-O34-C30
|
107.5144
|
|
O13-C9-H14
|
126.991
|
C18-C20-C24
|
117.8125
|
–
|
–
|
|
C3-C10-H11
|
111.0122
|
C18-C20-H25
|
121.4346
|
–
|
–
|
Table 1B: Optimized geometrical parameters of DBTI by DFT/ B3LYP with 6-311++G(d,p) basis set
|
Bond lengths (Å)
|
|
C1-C2
|
1.3997
|
C10-H12
|
1.0967
|
C24-H32
|
1.359
|
|
C1-C6
|
1.3939
|
C10-C14
|
1.5147
|
C26-H27
|
1.095
|
|
C1-H7
|
1.0832
|
C14-C15
|
1.3491
|
C26-C28
|
1.5455
|
|
C2-C3
|
1.3921
|
C15-H16
|
1.0893
|
C26-H31
|
1.0919
|
|
C2-H8
|
1.0851
|
C15-C17
|
1.4554
|
C28-H29
|
1.089
|
|
C3-C4
|
1.4029
|
C17-C18
|
1.4112
|
C28-H30
|
1.0936
|
|
C3-C10
|
1.5171
|
C17-C19
|
1.4152
|
C28-H32
|
1.4572
|
|
C4-C5
|
1.3896
|
C18-C20
|
1.3917
|
C33-H34
|
1.0948
|
|
C4-C9
|
1.4812
|
C18-H21
|
1.0803
|
C33-H35
|
1.0907
|
|
C5-C6
|
1.3917
|
C19-C22
|
1.3806
|
C33-C36
|
1.546
|
|
C5-C33
|
1.5076
|
C19-H23
|
1.0852
|
C36-H37
|
1.094
|
|
C6-O39
|
1.3649
|
C20-C24
|
1.39
|
C36-H38
|
1.0892
|
|
C9-O13
|
1.2216
|
C20-H25
|
1.0828
|
C36-O39
|
1.4579
|
|
C9-C14
|
1.4959
|
C22-C24
|
1.396
|
–
|
–
|
|
C10-H11
|
1.0969
|
C22-C26
|
1.5123
|
–
|
–
|
|
Bond angles (°)
|
|
C2-C1-C6
|
118.5211
|
H12-C10-C14
|
111.9372
|
C22-C26-C28
|
101.3739
|
|
C2-C1-H7
|
121.2674
|
C9-C14-C10
|
108.9942
|
C22-C26-H31
|
113.4203
|
|
C6-C1-H7
|
120.2114
|
C9-C14-C15
|
119.5279
|
H27-C26-C28
|
111.6235
|
|
C1-C2-C3
|
119.9287
|
C10-C14-C15
|
131.4776
|
H27-C26-H31
|
107.7282
|
|
C1-C2-H8
|
119.3749
|
C14-C15-H16
|
113.5279
|
C28-C26-H31
|
111.775
|
|
C3-C2-H8
|
120.6958
|
C14-C15-C17
|
131.868
|
C26-C28-H29
|
114.1682
|
|
C2-C3-C4
|
120.3155
|
H16-C15-C17
|
114.604
|
C26-C28-H30
|
111.7328
|
|
C2-C3-C10
|
128.7497
|
C15-C17-C18
|
124.5193
|
C26-C28-O32
|
106.6493
|
|
C4-C3-C10
|
110.9348
|
C15-C17-C19
|
117.4754
|
H29-C28-H30
|
109.266
|
|
C3-C4-C5
|
120.4952
|
C18-C17-C19
|
118.0053
|
H29-C28-O32
|
107.3348
|
|
C3-C4-C9
|
109.9704
|
C17-C18-C20
|
122.0958
|
H30-C28-O32
|
107.3437
|
|
C5-C4-C9
|
129.5343
|
C17-C18-H21
|
119.7491
|
C24-O32-C28
|
107.5129
|
|
C4-C5-C6
|
118.2297
|
C20-C18-H21
|
118.1546
|
C5-C33-H34
|
110.6631
|
|
C4-C5-C33
|
133.2551
|
C17-C19-C22
|
120.4133
|
C5-C33-H35
|
113.1536
|
|
C6-C5-C33
|
108.4696
|
C17-C19-H23
|
119.0843
|
C5-C33-C36
|
101.1394
|
|
C1-C6-C5
|
122.5085
|
C22-C19-H23
|
120.5024
|
H34-C33-H35
|
107.1137
|
|
C1-C6-O39
|
124.3126
|
C18-C20-C24
|
117.8164
|
H34-C33-C36
|
112.2093
|
|
C5-C6-O39
|
113.175
|
C18-C20-H25
|
121.4388
|
H35-C33-C36
|
112.6213
|
|
C4-C9-O13
|
126.6343
|
C24-C20-H25
|
120.7445
|
C33-C36-H37
|
111.603
|
|
C4-C9-C14
|
106.3681
|
C19-C22-C24
|
119.7754
|
C33-C36-H38
|
114.1268
|
|
O13-C9-C14
|
126.9976
|
C19-C22-C26
|
132.2866
|
C33-C36-O39
|
106.8638
|
|
C3-C10-H11
|
111.2344
|
C24-C22-C26
|
107.8829
|
H37-C36-H38
|
109.2168
|
|
C3-C10-H12
|
111.3211
|
C20-C24-C22
|
121.8918
|
H37-C36-O39
|
107.3892
|
|
C3-C10-C14
|
103.7313
|
C20-C24-O32
|
124.6749
|
H38-C36-O39
|
107.3122
|
|
H11-C10-H12
|
106.8163
|
C22-C24-O32
|
113.4303
|
C6-O39-C36
|
107.235
|
|
H11-C10-C14
|
111.8977
|
C22-C26-H27
|
110.9233
|
–
|
–
|
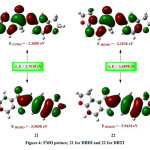
From the frontier molecular orbital (FMO) analysis the information about charge transfer within the molecule can be anticipated. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) is very crucial parameters for the investigation of quantum chemical parameters. Fig 4 (21) demonstrates HOMO-LUMO picture of DBDI and Fig 4 (22) is for DBTI. The charge transfer phenomenon supports the bioactive property of the molecule. The FMO study gives an understanding of the reactivity of the molecule and the active site can be established by the distribution of frontier orbital. The HOMO-LUMO study is also used for predicting the most reactive position in -electron systems and additionally explains several types of reactions in the conjugated frameworks. The less HOMO-LUMO energy gap suggests the molecule is softer and a large gap suggests a molecule is harder. The HOMO-LUMO gap in DBDI is 3.7018 eV and in DBTI is 3.6898 eV. Due to the low energy gap in the molecule DBDI, this molecule will have more ease in the charge transfer within the molecule than the molecule DBTI. This also suggests higher chemical reactivity. In the present study quantum chemical parameters have been established which provide good deal of comparison between these two molecules. Electronic parameters are tabulated in Table 2 and reactivity descriptors in Table 3. The ionization potential (I) provides information about ionization capability of the molecule whereas electron affinity (A) provides information regarding electron attraction capability. Our study reveals that ionization in DBTI is easier than DBDI; as former has less value of ionization potential. On the contradictory DBDI has more electron affinity than DBTI. The absolute hardness (η) and global softness (σ) corresponds to the HOMO-LUMO energy gap. The chemical potential (Pi) can be explored to determine the ionization capability of an electron. The global electrophilicity index (ω) provides idea about electrophilic character of the molecule. The electronic charge transfer is predicted by ΔNmax. The global reactivity descriptors’ study indicates both the molecules are strong electrophiles and therefor can undergo nucleophilic attack easily. The molecule DBDI involves more electron transfer than DBTI as predicted by ΔNmax; however it requires little bit more energy than DBTI.
Table 2: Electronic parameters
|
Entry
|
ETotal (a.u.)
|
EHOMO
(eV)
|
ELUMO
(eV)
|
ΔE
(eV)
|
I
(eV)
|
A
(eV)
|
|
DBDI
|
-845.0077
|
-5.9698
|
-2.2680
|
3.7018
|
5.9698
|
2.2680
|
|
DBTI
|
-997.6827
|
-5.9434
|
-2.2536
|
3.6898
|
5.9434
|
2.2536
|
Note: I = −EHOMO & A= −ELUMO
Table 3: Quantum chemical parameters
|
Entry
|
χ
(eV)
|
ɳ
(eV)
|
σ
(eV-1)
|
ω
(eV)
|
Pi
(eV)
|
ΔNmax
(eV)
|
Dipole moment (Debye)
|
|
DBDI
|
4.1189
|
1.8509
|
0.5403
|
4.5830
|
-4.1189
|
2.2553
|
3.5520
|
|
DBTI
|
4.0985
|
1.8449
|
0.5420
|
4.5525
|
-4.0985
|
2.2215
|
3.0116
|
Note: χ = (I + A)/2; ɳ = (I − A)/2; σ = 1/ɳ; ω = Pi2/2ɳ; Pi = −χ; ΔNmax = −Pi/ɳ
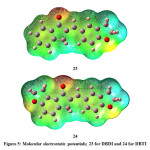
The MEP plots are displayed in Fig 5. The properties like dipole moment, electronegativity, partial charges and chemical reactivity of any molecule can be correlated with the aid of molecular electrostatic potential. The molecular electrostatic potential is a total charge distribution of a molecule space. The regions of positive, negative and neutral potentials are indicated by different colours. Red suggests a zone of negative electrostatic potential and the white zone of positive electrostatic potential. In the present case, it can be observed than negative electrostatic potential lies over oxygen atom in both DBDI and DBTI. On the other hand, the positive electrostatic potential is situated over hydrogen atoms of two aromatic rings. The blue part indicates zero electrostatic potential and it is mainly located over hydrogen atoms of dihydrofuran ring in both DBDI and DBTI. The molecule DBTI has less positive electrostatic potential than DBDI. This is due to the presence of extra dihydrofuran ring in DBTI. These zones of various electrostatic potential can give valuable data in regards to various sorts of intermolecular interactions and hence one can foresee the chemical behaviour of the molecule.
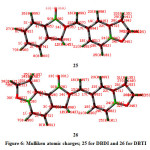
The different thermodynamic properties were computed from the theoretical vibrational frequencies and are presented in Table 6. The thermodynamic data disclosed in this could provide valuable insights to the other thermodynamic parameters. In present investigation, the theoretical thermodynamic calculations based on vibrational data provided valuable insights on thermodynamic stability of the title molecules. Our study reveals that the molecule DBTI is thermodynamically more stable than DBDI. Moreover DBTI possesses more degree of translational, rotational and vibrational freedom as compared to DBDI. Also, it has higher value of heat capacity. Mulliken atomic charges of DBDI and DBTI are presented in Table 4 and Fig 6. Mulliken atomic charges give important information regarding electropositive and electronegative nature of atoms. All hydrogen atoms are electropositive as they are attached to atoms having more electronegativity than hydrogen. Out of two oxygen atoms in DBDI molecule, 34O is more negative with Mulliken atomic charge value of -0.346342. On the other hand, 39O is the most negative with Mulliken atomic charge value of -0.357181.
Table 4: Mulliken atomic charges
|
DBDI
|
DBTI
|
|
Atom
|
Charge
|
Atom
|
Charge
|
|
1 C
|
-0.077564
|
1 C
|
-0.066467
|
|
2 C
|
-0.073918
|
2 C
|
-0.071827
|
|
3 C
|
-0.124734
|
3 C
|
-0.136440
|
|
4 C
|
-0.045291
|
4 C
|
-0.028203
|
|
5 C
|
-0.027585
|
5 C
|
-0.151259
|
|
6 C
|
-0.091625
|
6 C
|
0.237673
|
|
7 H
|
0.098412
|
7 H
|
0.103104
|
|
8 H
|
0.083048
|
8 H
|
0.081455
|
|
9 C
|
0.229546
|
9 C
|
0.228244
|
|
10 C
|
-0.099451
|
10 C
|
-0.097862
|
|
11 H
|
0.144775
|
11 H
|
0.141638
|
|
12 H
|
0.144745
|
12 H
|
0.141771
|
|
13 O
|
-0.317630
|
13 O
|
-0.330787
|
|
14 C
|
-0.235269
|
14 C
|
-0.230793
|
|
15 C
|
0.027322
|
15 C
|
0.025347
|
|
16 H
|
0.098993
|
16 H
|
0.097262
|
|
17 C
|
-0.093882
|
17 C
|
-0.092018
|
|
18 C
|
-0.050912
|
18 C
|
-0.050598
|
|
19 C
|
-0.034868
|
19 C
|
-0.035806
|
|
20 C
|
-0.081547
|
20 C
|
-0.081903
|
|
21 H
|
0.101597
|
21 H
|
0.102766
|
|
22 C
|
-0.171504
|
22 C
|
-0.171159
|
|
23 H
|
0.089924
|
23 H
|
0.089055
|
|
24 C
|
0.233928
|
24 C
|
0.233868
|
|
25 H
|
0.101132
|
25 H
|
0.101140
|
|
26 H
|
0.099328
|
26 C
|
-0.193460
|
|
27 H
|
0.098860
|
27 H
|
0.140271
|
|
28 C
|
-0.193507
|
28 C
|
-0.012403
|
|
29 H
|
0.140606
|
29 H
|
0.125239
|
|
30 C
|
-0.012580
|
30 H
|
0.125046
|
|
31 H
|
0.125473
|
31 H
|
0.134920
|
|
32 H
|
0.125273
|
32 O
|
-0.346495
|
|
33 H
|
0.135247
|
33 C
|
-0.165668
|
|
34 O
|
-0.346342
|
34 H
|
0.139763
|
|
–
|
–
|
35 H
|
0.143083
|
|
–
|
–
|
36 C
|
-0.012170
|
|
–
|
–
|
37 H
|
0.120278
|
|
–
|
–
|
38 H
|
0.120579
|
|
–
|
–
|
39 O
|
-0.357181
|
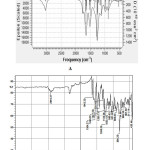
The theoretical IR spectrum of DBTI is computed at DFT/B3LYP method using 6-311++G (d,p) basis set. The theoretical and experimental IR spectra are Figure 7A and Figure 7B respectively. There are total 111 fundamental modes of vibrations as per 3N-6 formula. The important experimental IR vibrations are 2916.37, 1643.35, 1564.27, 1490.97, and 1427.32 cm-1. The 2916.37 cm-1 is due to the asymmetric C-H vibrations. The similar vibration is at 2917.36 cm-1 in the theoretical spectrum. The C=O frequency is appeared at 1643.35 cm-1. The carbonyl stretching is located at 1679.68 cm-1 in the simulated IR spectrum. The C=C vibrations are 1564.27, 1490.97, and 1427.32 cm-1. There is good agreement between the theoretical and experimental IR spectra.
Table 5: Thermodynamic properties
|
Parameter
|
DBDI
|
DBTI
|
|
E total (kcal mol-1)
Translational
Rotational
Vibrational
|
179.528
0.889
0.889
177.751
|
205.961
0.889
0.889
204.183
|
|
Heat Capacity at constant volume, Cv (cal mol-1K-1)
Translational
Rotational
Vibrational
|
61.812
2.981
2.981
55.850
|
71.567
2.981
2.981
65.605
|
|
Total entropy S (cal mol-1K-1)
Translational
Rotational
Vibrational
|
128.401
42.590
34.076
51.735
|
140.894
43.033
34.964
62.897
|
|
Zero point Vibrational Energy Ev0 (kcal mol-1)
|
169.82521
|
194.65241
|
|
Rotational constants (GHZ):
|
1.2333834 0.1314400 0.1190901
|
1.0102184 0.0921864 0.0847426
|
|
E (RB3LYP) (a.u.)
|
-845.0077
|
-997.6827
|
Conclusions
In the present research work, DFT/B3LYP method at 6-311++G (d,p) basis set has been used for the exploration of various important structural, electronic and quantum chemical parameters of title molecules. An elaborative correlation among DBDI and DBTI has been presented. The properties like the HOMO-LUMO energy gap, charge transfer phenomenon, molecular electrostatic potential, and global reactivity descriptors have been explored using the same level of method. Additionally, important thermodynamic statistics have been computed and these two molecules have been compared based on this data to elucidate their thermodynamic behaviour. The theoretical analysis of the two title compounds furnished the following results. Both DBDI and DBTI possess C1 point group symmetry due to the overall asymmetry of the molecules. The molecule DBDI possesses a higher dipole moment than the molecule DBTI. The HOMO-LUMO gap in DBDI is 3.7018 eV and in DBTI is 3.6898 eV. The MEP analysis suggests that the molecule DBTI has less positive electrostatic potential than DBDI. Thermochemical data reveals that the molecule DBTI is thermodynamically more stable than DBDI. Moreover, DBTI possesses more degree of translational, rotational, and vibrational freedom as compared to DBDI. The 39O is the most negative with Mulliken atomic charge value of -0.35718 in the DBTI molecule whereas 34O is more negative with Mulliken atomic charge value of -0.346342 in DBDI.
Acknowledgments
Authors acknowledge Central instrumentation facility, Savitribai Phule Pune University, Pune for NMR and Mass spectral analysis. Authors also would like to thank Lokenete Vyankatrao Hiray Arts, Science and Commerce College Panchavati, Nashik and Arts, Science and Commerce College, Manmad for permission and providing necessary research facilities. Authors would like to express their sincere and humble gratitude to Prof. Arun B. Sawant for Gaussian study. Dr. Aapoorva Hiray, Coordinator, MG Vidyamandir institute, is gratefully acknowledged for Gaussian package.
Funding
We have not received any kind of fund for the research work.
Conflict of Interest
Authors declared that he do not have any conflict of interest regarding this research article.
References
- Patil, S.A., Patil, R. and Patil, S.A., 2017. Recent developments in biological activities of indanones. European journal of medicinal chemistry, 138, pp.182-198.
CrossRef
- Menezes, J.C., 2017. Arylidene indanone scaffold: medicinal chemistry and structure–activity relationship view. RSC advances, 7(15), pp.9357-9372.
CrossRef
- Singh, A., Fatima, K., Singh, A., Behl, A., Mintoo, M.J., Hasanain, M., Ashraf, R., Luqman, S., Shanker, K., Mondhe, D.M. and Sarkar, J., 2015. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. European Journal of Pharmaceutical Sciences, 76, pp.57-67.
CrossRef
- Tang, M.L., Zhong, C., Liu, Z.Y., Peng, P., Liu, X.H. and Sun, X., 2016. Discovery of novel sesquistilbene indanone analogues as potent anti-inflammatory agents. European journal of medicinal chemistry, 113, pp.63-74.
CrossRef
- Panara, M.R., Greco, A., Santini, G., Sciulli, M.G., Rotondo, M.T., Padovano, R., di Giamberardino, M., Cipollone, F., Cuccurullo, F., Patrono, C. and Patrignani, P., 1995. Effects of the novel anti‐inflammatory compounds, N‐[2‐(cyclohexyloxy)‐4‐nitrophenyl] methanesulphonamide (NS‐398) and 5‐methanesulphonamido‐6‐(2, 4‐difluorothiophenyl)‐1‐indanone (L‐745, 337), on the cyclo‐oxygenase activity of human blood prostaglandin endoperoxide synthases. British journal of pharmacology, 116(5), pp.2429-2434.
CrossRef
- Gómez, N., Santos, D., Vázquez, R., Suescun, L., Mombrú, Á., Vermeulen, M., Finkielsztein, L., Shayo, C., Moglioni, A., Gambino, D. and Davio, C., 2011. Synthesis, Structural Characterization, and Pro‐apoptotic Activity of 1‐Indanone Thiosemicarbazone Platinum (II) and Palladium (II) Complexes: Potential as Antileukemic Agents. ChemMedChem, 6(8), pp.1485-1494.
CrossRef
- Saravanan, V.S., Selvan, P.S., Gopal, N. and Gupta, J.K., 2006. Synthesis and pharmacological evaluation of some indanone-3-acetic acid derivatives. Asian Journal of Chemistry, 18(4), p.2597.
- Li, C.S., Black, W.C., Chan, C.C., Ford-Hutchinson, A.W., Gauthier, J.Y., Gordon, R., Guay, D., Kargman, S. and Lau, C.K., 1995. Cyclooxygenase-2 inhibitors. Synthesis and pharmacological activities of 5-methanesulfonamido-1-indanone derivatives. Journal of medicinal chemistry, 38(25), pp.4897-4905.
CrossRef
- Hammen, Philip D., and George M. Milne Jr. “2-Aminomethyleneindanone analgesic agents.” U.S. Patent 4,164,514, issued August 14, 1979.
- Sheng, R., Xu, Y., Hu, C., Zhang, J., Lin, X., Li, J., Yang, B., He, Q. and Hu, Y., 2009. Design, synthesis and AChE inhibitory activity of indanone and aurone derivatives. European journal of medicinal chemistry, 44(1), pp.7-17.
CrossRef
- Albrecht, R., Kessler, H.J. and Schroder, E., Bayer Pharma AG, 1972. Antimicrobial indanones. U.S. Patent 3,671,520.
- Omran, Z., Cailly, T., Lescot, E., Sopkova-de Oliveira Santos, J., Agondanou, J.H., Lisowski, V., Fabis, F., Godard, A.M., Stiebing, S., Le Flem, G. and Boulouard, M., 2005. Synthesis and biological evaluation as AChE inhibitors of new indanones and thiaindanones related to donepezil. European journal of medicinal chemistry, 40(12), pp.1222-1245.
CrossRef
- Herzog, M.N., Chien, J.C. and Rausch, M.D., 2002. Novel 2-indenyl ansa-zirconocenes for the polymerization of α-olefins. Journal of organometallic chemistry, 654(1-2), pp.29-35.
CrossRef
- DJ, K., 2007. Hamel E. Jung MK. Flynn BL. Bioorg. Med. Chem, 15, p.3290.
CrossRef
- Leoni, L.M., Hamel, E., Genini, D., Shih, H., Carrera, C.J., Cottam, H.B. and Carson, D.A., 2000. Indanocine, a microtubule-binding indanone and a selective inducer of apoptosis in multidrug-resistant cancer cells. Journal of the National Cancer Institute, 92(3), pp.217-224.
CrossRef
- Patel, V., Bhatt, N., Bhatt, P. and Joshi, H.D., 2014. Synthesis and pharmacological evaluation of novel 1-(piperidin-4-yl)-1H-benzo [d] imidazol-2 (3H)-one derivatives as potential antimicrobial agents. Medicinal Chemistry Research, 23(4), pp.2133-2139.
CrossRef
- Sheridan, H., Frankish, N. and Farrell, R., 1999. Synthesis and antispasmodic activity of analogues of natural pterosins. European journal of medicinal chemistry, 34(11), pp.953-966.
CrossRef
- Syrchina, A.I. and Semenov, A.A., 1982. Natural indanones. Chemistry of Natural Compounds, 18(1), pp.1-11.
CrossRef
- Jaki, B., Heilmann, J. and Sticher, O., 2000. New Antibacterial Metabolites from the Cyanobacterium Nostoc c ommune (EAWAG 122b). Journal of Natural Products, 63(9), pp.1283-1285.
CrossRef
- Nagle, D.G., Zhou, Y.D., Park, P.U., Paul, V.J., Rajbhandari, I., Duncan, C.J. and Pasco, D.S., 2000. A New Indanone from the Marine Cyanobacterium Lyngbya m ajuscula That Inhibits Hypoxia-Induced Activation of the VEGF Promoter in Hep3B Cells. Journal of natural products, 63(10), pp.1431-1433.
CrossRef
- Kovganko, N.V., Kashkan, Z.N. and Krivenok, S.N., 2004. Bioactive Compounds of the Flora of Belarus. 4. Pterosins A and B from Pteridium aquilinum. Chemistry of natural compounds, 40(3).
CrossRef
- Ge, X., Ye, G., Li, P., Tang, W.J., Gao, J.L. and Zhao, W.M., 2008. Cytotoxic diterpenoids and sesquiterpenoids from Pteris multifida. Journal of natural products, 71(2), pp.227-231.
CrossRef
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2019. Aqua‐mediated rapid and benign synthesis of 1,2,6,7‐tetrahydro‐8H‐indeno [5,4‐b] furan‐8‐one‐appended novel 2‐arylidene indanones of pharmacological interest at ambient temperature. Journal of the Chinese Chemical Society.67:306-315.
CrossRef
- Chen, Z., Pitchakuntla, M. and Jia, Y., 2019. Synthetic approaches to natural products containing 2,3-dihydrobenzofuran skeleton. Natural product reports, 36(4), pp.666-690.
CrossRef
- Dawood, K.M., 2013. Benzofuran derivatives: a patent review. Expert opinion on therapeutic patents, 23(9), pp.1133-1156.
CrossRef
- Keylor, M.H., Matsuura, B.S. and Stephenson, C.R., 2015. Chemistry and biology of resveratrol-derived natural products. Chemical reviews, 115(17), pp.8976-9027.
CrossRef
- Khanam, H., 2015. Shamsuzzaman Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem, 97(1), pp.483-504.
CrossRef
- Naik, R., Harmalkar, D.S., Xu, X., Jang, K. and Lee, K., 2015. Bioactive benzofuran derivatives: Moracins A–Z in medicinal chemistry. European journal of medicinal chemistry, 90, pp.379-393.
CrossRef
- Radadiya, A. and Shah, A., 2015. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. European journal of medicinal chemistry, 97, pp.356-376.
CrossRef
- Liang, Z., Xu, H., Tian, Y., Guo, M., Su, X. and Guo, C., 2016. Design, synthesis and antifungal activity of novel benzofuran-triazole hybrids. Molecules, 21(6), p.732.
CrossRef
- Kenchappa, R., Bodke, Y.D., Chandrashekar, A., Telkar, S., Manjunatha, K.S. and Sindhe, M.A., 2017. Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-substituted phenyl) pyridine derivatives as potent biological agents. Arabian Journal of Chemistry, 10, pp.S1336-S1344.
CrossRef
- Aswathanarayanappa, C., Bheemappa, E., Bodke, Y.D., Bhovi, V.K., Ningegowda, R., Shivakumar, M.C., Peethambar, S.K. and Telkar, S., 2013. 5-phenyl-1-benzofuran-2-yl derivatives: synthesis, antimicrobial, and antioxidant activity. Medicinal Chemistry Research, 22(1), pp.78-87.
CrossRef
- Abdel-motaal, M., Kandeel, E. M., Abou-Elzahab, M., & Elghareeb, F. (2017). Synthesis and Evaluation of Antioxidant Activity of Some New Heterocyclic Compounds Bearing the Benzo[B]Furan Moiety. European Scientific Journal, ESJ, 13(30), 297-213.
CrossRef
- Hiremathad, A., Chand, K. and Keri, R.S., 2018. Development of coumarin–benzofuran hybrids as versatile multitargeted compounds for the treatment of Alzheimer’s Disease. Chemical biology & drug design, 92(2), pp.1497-1503.
CrossRef
- Thévenin, M., Thoret, S., Grellier, P. and Dubois, J., 2013. Synthesis of polysubstituted benzofuran derivatives as novel inhibitors of parasitic growth. Bioorganic & medicinal chemistry, 21(17), pp.4885-4892.
CrossRef
- Rizzo, S., Rivière, C., Piazzi, L., Bisi, A., Gobbi, S., Bartolini, M., Andrisano, V., Morroni, F., Tarozzi, A., Monti, J.P. and Rampa, A., 2008. Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, β amyloid aggregation, and Aβ neurotoxicity. Journal of medicinal chemistry, 51(10), pp.2883-2886.
CrossRef
- Xie, Y.S., Kumar, D., Bodduri, V.V., Tarani, P.S., Zhao, B.X., Miao, J.Y., Jang, K. and Shin, D.S., 2014. Microwave-assisted parallel synthesis of benzofuran-2-carboxamide derivatives bearing anti-inflammatory, analgesic and antipyretic agents. Tetrahedron Letters, 55(17), pp.2796-2800.
CrossRef
- Asif, M., 2016. Mini review on important biological properties of benzofuran derivatives. J Anal Pharm Res, 3(2). 48-50.
CrossRef
- Heravi, M.M., Zadsirjan, V., Hamidi, H. and Amiri, P.H.T., 2017. Total synthesis of natural products containing benzofuran rings. RSC advances, 7(39), pp.24470-24521.
CrossRef
- Kamal, M., Shakya, A.K. and Jawaid, T., 2011. Benzofurans: a new profile of biological activities. International Journal of Medical and Pharmaceutical Sciences, 1(3), pp.1-15.
CrossRef
- Adole, V.A., Pawar, T.B., Koli, P.B. and Jagdale, B.S., 2019. Exploration of catalytic performance of nano-La 2 O 3 as an efficient catalyst for dihydropyrimidinone/thione synthesis and gas sensing. Journal of Nanostructure in Chemistry, 9(1), pp.61-76.
CrossRef
- Adole, V.A., More, R.A., Jagdale, B.S., Pawar, T.B. and Chobe, S.S., 2020. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2‐(2‐Hydrazineyl) thiazole Derivatives. ChemistrySelect, 5(9), pp.2778-2786.
CrossRef
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sagane, A.A., 2020. Ultrasound promoted stereoselective synthesis of 2, 3-dihydrobenzofuran appended chalcones at ambient temperature. South African Journal of Chemistry, 73, pp.35-43.
CrossRef
- Lade, J.J., Patil, B.N., Vhatkar, M.V., Vadagaonkar, K.S. and Chaskar, A.C., 2017. An Efficient Synthesis of Pyrrolo [1,2‐a] quinoxalines by Copper‐Catalyzed C− H Activation of Arylacetic Acids. Asian Journal of Organic Chemistry, 6(11), pp.1579-1583.
CrossRef
- Patil, B.N., Sathe, P.A., Parade, B.S., Vadagaonkar, K.S. and Chaskar, A.C., 2018. Transition metal free one pot synthesis of aryl carboxylic acids via dehomologative oxidation of styrenes. Tetrahedron letters, 59(49), pp.4340-4343.
CrossRef
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sawant, A.B., Experimental and theoretical exploration on single crystal, structural, and quantum chemical parameters of (E)‐7‐(arylidene)‐1,2,6,7‐tetrahydro‐8H‐indeno [5,4‐b] furan‐8‐one derivatives: A comparative study. Journal of the Chinese Chemical Society. https://doi.org/10.1002/jccs.202000006.
CrossRef
- Patil, M.P., Singh, R.D., Koli, P.B., Patil, K.T., Jagdale, B.S., Tipare, A.R. and Kim, G.D., 2018. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microbial pathogenesis, 121, pp.184-189.
CrossRef
- Shinde, S.G., Patil, M.P., Kim, G.D. and Shrivastava, V.S., 2020. Ni, C, N, S multi-doped ZrO2 decorated on multi-walled carbon nanotubes for effective solar induced degradation of anionic dye. Journal of Environmental Chemical Engineering, 8(3), p.103769.
CrossRef
- Lade, J.J., Patil, B.N., Vadagaonkar, K.S. and Chaskar, A.C., 2017. Oxidative cyclization of amidoximes and thiohydroximic acids: A facile and efficient strategy for accessing 3, 5-disubstituted 1, 2, 4-oxadiazoles and 1, 4, 2-oxathiazoles. Tetrahedron Letters, 58(22), pp.2103-2108.
CrossRef
- Chaudhari, M.B., Jayan, K. and Gnanaprakasam, B., 2020. Sn-Catalyzed Criegee-Type Rearrangement of Peroxyoxindoles Enabled by Catalytic Dual Activation of Esters and Peroxides. The Journal of Organic Chemistry, 85(5), pp.3374-3382.
CrossRef
- Koli, P.B., Kapadnis, K.H., Deshpande, U.G. and Patil, M.R., 2018. Fabrication and characterization of pure and modified Co3O4 nanocatalyst and their application for photocatalytic degradation of eosine blue dye: a comparative study. Journal of Nanostructure in Chemistry, 8(4), pp.453-463.
CrossRef
- Shinde, S.G., Patil, M.P., Kim, G.D. and Shrivastava, V.S., 2019. Multi-doped ZnO photocatalyst for solar induced degradation of indigo carmine dye and as an antimicrobial agent. Journal of Inorganic and Organometallic Polymers and Materials, pp.1-12.
CrossRef
- Koli, P.B., Kapadnis, K.H. and Deshpande, U.G., 2019. Transition metal decorated Ferrosoferric oxide (Fe3O4): An expeditious catalyst for photodegradation of Carbol Fuchsin in environmental remediation. Journal of Environmental Chemical Engineering, 7(5), p.103373.
CrossRef
- Adole, V.A., Synthetic approaches for the synthesis of dihydropyrimidinones/ thiones (biginelli adducts): a concise review. 9 (6) 1067-1091.
- Adole, V.A., Waghchaure, R.H., Jagdale, B.S. and Pawar, T.B., 2020. Investigation of Structural and Spectroscopic Parameters of Ethyl 4-(4-isopropylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: a DFT Study. Chemistry & Biology Interface, 10(1), 22-20.
- Sawant, A.B. and Wagh, S.G., 2018. Density, viscosity, DFT and FTIR study of tertiary butyl alcohol and ethanol with DMSO and DMF at room temperature. Indian Journal of Pure & Applied Physics (IJPAP), 56(5), pp.405-414.
- Pawar, T.B., Jagdale, B.S., Sawant, A.B. and Adole, V.A., 2017. DFT Studies of 2-[(2-substitutedphenyl) carbamoyl] benzoic acids. Journal of Chemical, Biological and Physical Sciences, 7, pp.167-175.
- Seyedkatouli, S., Vakili, M., Tayyari, S.F., Hansen, P.E. and Kamounah, F.S., 2019. Molecular structure and intramolecular hydrogen bond strength of 3-methyl-4-amino-3-penten-2-one and its NMe and N-Ph substitutions by experimental and theoretical methods. Journal of Molecular Structure, 1184, pp.233-245.
CrossRef
- Adole, V.A., Waghchaure, R.H., Jagdale, B.S., Pawar, T.B. and Pathade, S.S., Molecular tructure, frontier molecular orbital and spectroscopic examination on dihydropyrimidinones: a comparative computational approach Journal of Advanced Scientific Research, 2020. 11 (2), pp.64-70.
- Selvaraj, S., Rajkumar, P., Kesavan, M., Thirunavukkarasu, K., Gunasekaran, S., Devi, N.S. and Kumaresan, S., 2020. Spectroscopic and structural investigations on modafinil by FT-IR, FT-Raman, NMR, UV–Vis and DFT methods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 224, p.117449.
CrossRef
- Balakrishnan, A., Sambanthan, M., Murugesan, R. and Sundaram, S., 2020. Molecular structure, spectroscopic (FT-IR, FT-Raman, NMR, UV-VIS), chemical reactivity and biological examinations of Ketorolac. Journal of Molecular Structure, p.128040.
CrossRef
- Manjusha, P., Muthu, S. and Raajaraman, B.R., 2020. Density functional studies and spectroscopic analysis (FT-IR, FT-Raman, UV–visible, and NMR) with molecular docking approach on an antifibrotic drug Pirfenidone. Journal of Molecular Structure, 1203, p.127394.
CrossRef
- Simbizi, R., Gahungu, G. and Nguyen, M.T., 2020. Molecular structure, IR, Raman and UV–VIS spectra of 2-cyanothiophene and 3-cyanothiophene: A comparative quantum chemical investigation. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, p.118393.
CrossRef
- Frisch, M., Frisch, M., Trucks, G., Schlegel, K.H., Scuseria, G., Robb, M.A., Cheeseman, J., Montgomery, J., Vreven, T., Kudin, K.N. and Burant, J., 2004. Gaussian 03, revision D. 02.
Views: 1,192
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Vishnu Ashok Adole2
, Vishnu Ashok Adole2 , Bapu Sonu Jagdale,1,2*
, Bapu Sonu Jagdale,1,2* , Thansing Bhavsing Pawar1
, Thansing Bhavsing Pawar1 , Bhatu Shivaji Desale2 and Rohit Shankar Shinde2
, Bhatu Shivaji Desale2 and Rohit Shankar Shinde2 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal