Soumya Mukherjee1* , Mohammed Shahnawaz2 and Sathi Banerjee3
, Mohammed Shahnawaz2 and Sathi Banerjee3
1Department of Metallurgical Engineering, Kazi Nazrul University, Asansol, India, 713340,
2Department of Electronics and Telecommunication Engineering, Heritage Institute of Technology, Kolkata, India, 700107
3Department of Metallurgical and Materials Engineering, Jadavpur University, Kolkata, India, 700032
Corresponding Author Email: smmukherjee3@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170205
Article Publishing History
Article Received on : 15 Aug 2020
Article Accepted on : 30 Aug 2020
Article Published : 30 Aug 2020
Plagiarism Check: Yes
Reviewed by: Sumit Desai
Second Review by: Tushar Ashok Kshirsagar
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
Barium bismuth titanate materials are noted for ferroelectric behavior, competitor to lead based relaxor dielectrics and even for coating having wear resistance, toughness for improving surface engineering applications. In the present context, a modified solid state process aided by agate-mortar activation for milling is carried in compare to mechanochemical milling, melt-quench method to generate the complex ceramic structure. Molar ratio of BaO:Bi2O3:TiO2 1:2:4 ratio have been taken with fixed soaking temperature to develop the material. Activation milling period is varied for 20 hours, 25 hours and 30 hours while soaking period descends in the order 10 hours, 8 hours and 6 hours keeping fixed temperature of about 700°C. XRD confirms the presence of peaks for all cases. Crystallite size is estimated by Scherrers formula with proper planes of index corresponding to JCPDS data. FTIR confirmed the phases developed by XRD while indicating the proper M-O bond formation from analysis in the required spectral range. EDX spectra analysis given the presence of required elements present in the sample.
KEYWORDS:
Aurivillius; Barium Bismuth Titanate; Bonding; Morphology; Phase;
Copy the following to cite this article:
Mukherjee S, Shahnawaz M, Banerjee S. Phase Development of Barium Bismuth Titanate by Modified Solid State Route. Mat. Sci. Res. India; 17(2).
|
Copy the following to cite this URL:
Mukherjee S, Shahnawaz M, Banerjee S. Phase Development of Barium Bismuth Titanate by Modified Solid State Route. Mat. Sci. Res. India; 17(2). Available from: https://bit.ly/2R4TjO8
|
Introduction
The family of bismuth oxides has been discovered by Aurivillius and the properties are found to be interest for applications as temperature stable piezo ferroelectrics. Bismuth layered crystal structures have been studied in details since majority of them have normal ferroelectrics with fairly high Curie temperature. Barium bismuth titanate is an aurivillus based ceramic compound which consists of n number of perovskite like (An-1BnO3n+3)2- unit cell sandwiched between (Bi2O2)2+ layers along pseudo tetragonal c axis. A site having 12 fold coordination of perovskite is occupied by large cations like Na+, K+, Ca2+, Sr2+, Ba2+ and others while B site having 6 fold coordinations is occupied by small cations like Fe3+, Cr3+, Ti4+, Nb5+ or W6+.1, 2, 3 Electric properties are noted to be anisotropic with maximum value of conductivity and major component of polarization to be parallel to (Bi2O2)2- layers. Properties of these polycrystalline materials are influenced by microstructure especially by orientation of plate like grains and by length to thickness ratio of grains. It is also noted that these bismuth layered ferroelectric structures exhibit anisotropy with high value of piezoelectric constant along a, b axis but not along c-axis. In some cases crystal may be oriented in some specific direction to achieve better properties.4, 5 Barium bismuth titanate is found to be applicable for high temperature piezoelectric sensors, non-volatile ferroelectric random access memory devices. In case of Bismuth layered ferroelectric, (Bi2O2)2+ acts as insulating paraelectric layer which control the conductivity while the perovskite unit cell contributes to ferroelectricity.1, 6 Material is also found to be suitable for relaxor ferroelectrics with high dielectric, piezoelectric response over wide temperature range.5 Material is found to be synthesized by solid state process and chemical route. Several methods like polymer precursor pechini process, sol-gel, hydrothermal, co-precipitation, mechanochemical synthesis have been carried to synthesize the material.1, 2, 7, 8 BBIT powders can also prepared by conventional route using BaCO3, TiO2 and Bi2O3 as precursor but the problem of inhomogenity in stoichometric ratio persists. Moreover there will be undesirable loss at high temperature of Bi content due to volatility of Bi2O3.9, 10, 12 In the present context, modified solid state process assisted by agate mortar mill activation is carried. The synthesized material was analyzed by XRD to determine the phases formed followed by FTIR analysis to determine bonding and vibration of M-O coordinations followed by EDX analyses on selected areas of SEM morphology.
Materials and Methods
The precursor materials required for synthesis were AR grade Barium oxide, Bismuth Oxide and Titania. Initially, precursors were taken in proper molar ratio of BaO:Bi2O3:TiO2 in 1:2:4 molar ratio and then milled in agate mortar for mechanochemical activations. Milling was carried for 20 hours, 25 hours and 30 hours respectively. After milling powder samples were put into tubular furnace in presence of air atmosphere for annealing. Annealing was carried at 700°C for 10 hours, 8 hours and 6 hours soaking period respectively after milling respectively the powder samples for 20 hours, 25 hours and 30 hours respectively. Phase analysis was carried by XRD (Rigaku Ultima III) with with Cu Kα radiation whose operating condition was 40 KV, 40 mA current rating with scanning rate of 1°/min and in the range 2θ = 10-80°. For FTIR spectroscopy (IR-Prestige 21, Shimadzu) the sample was prepared by adding KBr to the sample to make a pellet by hydraulic pressure under pressure of 6t/cm2. FTIR normal scan was carried in the range of 4500cm-1 to 450cm-1 in air atmosphere without any purging of gaseous atmosphere to maintain conditioned atmosphere. One KBr pellet was also made as the reference material for carrying the scan of the powder sample. EDX analysis (Oxford Instruments) was carried to obtain chemical analysis of the sample along with morphology of particular section of powder samples after putting the powder sample on carbon tape with gold coating for better observation without any static charging effect.
Results and Discussion
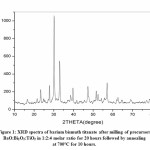
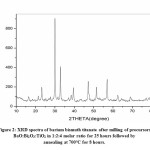
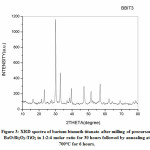
Fig 1-3 represents XRD spectra of Barium Bismuth Titanate. In all cases annealing temperature is kept fixed while with increase in milling activation period soaking time at fixed annealing temperature is decreased. As per JCPDS card no PDF# 00-035-0757 all the peaks corresponds to aurivillius BaBi4Ti4O15 for all samples at three corresponding conditions. None of the samples exhibit intermediate phase or presence of precursor oxides after annealing. Crystallite size is estimated using Scherrer’s formula t=0.9λ/βCosθ where λ is the wavelength, β is full width half mean and θ is the angle in degrees. Crystallite size for Fig 1 is estimated to be about 35.11nm while for Fig 2 and Fig 3 it is estimated to be about 35.08nm and 34.73 nm respectively. Thus, it is noted for the present experimental condition with increase in milling duration and decrease in soaking period at constant annealing temperature crystallite size decreases. All XRD spectra indicate crystalline peaks with sharp intensity without any humps or deconvulation of peaks. Three major planes of orientation (101), (109) and (110) planes are noted for all cases whilst the highest intensity peak is indexed along (109) planes.
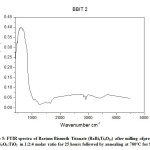
FTIR analysis is carried in the scan range of 450-4500cm-1 using KBr pellet as standard for reference. For all samples nature of graph is found to be similar. The bands observed in the range of 800-1100cm-1 is due to presence of Si-O-Si bonds. Approximately at about 1091cm-1, 1166cm-1 for Fig 4 is due to Si-O-Si bonds while such peaks are noted at about 1087cm-1,1162cm-1 and at about 1078cm-1,1166cm-1 for Fig 5 and Fig 6 respectively. As per literature bands observed near 858, 900, 970, and 1036 cm-1 are due to the presence of Si–O–Si bonds. [11, 12] The band at about 860cm-1 is due to bending vibration of Si-O-Si linkage while bands observed at about 920 and 970cm-1 are mainly due to symmetric stretching vibration of Si-O units. The spectra within 480-680cm-1 represents Bi-O bonds. Absorption at about 500cm-1 is due to BiO6 distorted octahedral units. [11, 12] In the present context, absorption spectra at about 542cm-1, 567cm-1, 563cm-1, 579cm-1,617cm-1,667cm-1, 675cm-1 is mainly due to Bi-O bonds. Ti-O bonds are reflected at about 730cm-1 which actually corresponds to asymmetric stretching of Ti-O tetrahedral units.
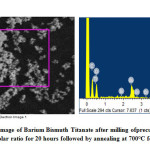
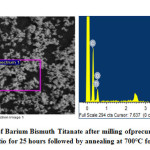
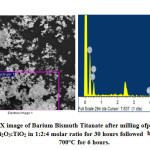
Figures 7-9 represents the morphological features studied by SEM followed by EDX analysis at certain specific zone. The morphology is noted to have agglomeration while the agglomerated chunks have irregular shape. Individual particulates are having spherical to irregular polygon shape with few interconnected pores. Particulates are about 0.5μ while rough patches are noted in some discreet portions. EDX analysis represents presence of Ba, Bi, Ti and O as elements required for the formation of barium bismuth titanate. EDX analysis in addition to FTIR bond stretching and vibration validates the phase analysis by XRD. Thus EDX provides the chemical analyses in designated morphological portions by means of presence of only desired peaks of the required elements.
Conclusions
Modified Solid state route was carried to successfully synthesized barium bismuth titanate after undergoing agate mortar milling activation. Milling activation was carried for 20, 25 and 30 hours followed by annealing at 700°C for 10 hrs, 8 hours and 6 hours respectively. Crystallite size was estimated to be about 35.11nm, 35.08nm and 34.73 nm respectively. Crystallite size decreases with increase in milling time while the soaking period decreases at constant annealing temperature. FTIR analysis exhibits the required M-O coordinations of Si-O-Si bonds, Bi-O bonds and Ti-O bonds respectively. Morphology by SEM exhibits agglomeration tendency while individual particulates were noted to be spherical to irregular polygonal shape. EDX analysis confirms the presence of Ba, Bi, Ti and O as elements required for the formation of aurivillius compound.
Acknowledgements
Author is highly greatful to Department of Metallurgical & Materials Engineering, Jadavpur University for providing characterization facilities like XRD, SEM-EDX and FTIR.
Conflict of Interest
There is no conflict of interest among authors.
Funding Sources
There is no funding from any agency for carrying the experimental research.
References
- J.D. Bobić, M.M. Vijatović, T. Rojac, B. D. Stojanović, Characterization and properties of barium bismuth titanate. Process. Appl. Ceram. 3, [1-2] 9-12 (2009)
CrossRef
- Z. ž. Lazarevic, J. Bobić, N.ž. Romčević, N. Paunović, B. D. Stojanović, Study of Barium Bismuth titanate prepared by Mechanochemical synthesis,. Sci. Sinter. 41, 329-335 (2009)
CrossRef
- J.D. Bobić, B. D. Stojanović, C.O. Paiva Santos, L.J. Zivkovic, M.M. Vijatović and M. Cilense, Structure and properties of Barium Bismuth titanate prepared by Mechanochemical synthesis. Ferroelectrics, 368, 145-153 (2008)
CrossRef
- S. Tanaka, Y. Tomita, R. Furushima, H. Shimizu, Y. Doshida, K. Uematsu, Fabrication of crystal oriented barium bismuth titanate ceramics in high magnetic field and subsequent reaction sintering. Sci. Technol. Adv. Mater. 10, 014602 (2009)
CrossRef
- J.D. Bobić, M.M. Vijatović, S. Greičius, J Banys and B. D. Stojanović, Dielectric and relaxation behaviour of BaBi4Ti4O15 ceramics. J. Alloys. Compds. 499, 221–226 (2010)
CrossRef
- J.D. Bobić, R.M.Katiliute, M. Ivanov, N.I. Illić, A.S. Dzunuzović, M.M. Vijatović Petrović, J. Banys, B. D. Stojanović, Influence of tungsten doping on dielectric, electrical and ferroelectric behavior of BaBi4Ti4O15 ceramics. J. Alloys. Compds. 702, 619–625 (2017)
CrossRef
- A.V. Murugan, S.C. Navale, V. Ravi, Preparation of nanocrystalline ferroelectric BaBi4Ti4O15 by Pechini method. Mater. Lett. 60, 1023-1025 (2006)
CrossRef
- D. Xie, W. Pan, Study of BaBi4Ti4O15 nanscaled powder by sol-gel method. Mater. Lett. 57, 2970-2974 (2003)
CrossRef
- B.J. Kennedy, Y. Kubota, B.A. Hunter, Ismunandar, K. Kato, Structural phase transition in the layered bismuth oxide BaBi4Ti4O15. Solid. State. Comm. 126, 653-658 (2003)
CrossRef
- C. Jovalekic, M. Pavlovic, O. Osmokriovic, Lj. Atanasoska, X –ray photoelectron spectroscopy study of Bi4Ti3O12 ferroelectric ceramics. Appl. Phys. Lett. 72, 1051-1053, (1998)
CrossRef
- A.R. Molla, A. Tarafder, B. Karmakar, Fabrication and properties of Nd3+ doped ferroelectric barium bismuth titanate glass ceramic nanocomposites. J. Alloys. Compds. 680, 237–246 (2016)
CrossRef
- A.R. Molla, A. Tarafder, Siddhartha Mukherjee, S.K. Mohanty, B. Karamakar, Processing and Properties of Eu3+ Doped Barium Bismuth Titanate (BaBi4Ti4O15)glass-ceramics nanocomposites. J. Am. Ceram. Soc. 96, [8] 2387-2395 (2013)
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Mohammed Shahnawaz2 and Sathi Banerjee3
, Mohammed Shahnawaz2 and Sathi Banerjee3 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal