Rajesh Kumar Meena1* , Risikesh Meena2
, Risikesh Meena2 , Dinesh Kumar Arya3
, Dinesh Kumar Arya3 , Sapana Jadoun4
, Sapana Jadoun4 , Renu Hada5
, Renu Hada5 and Roopa Kumari6
and Roopa Kumari6
1Department of Chemistry, Kalindi College, University of Delhi, Delhi, India,110008
2Department of Botany, University of Rajasthan, Jaipur, India, 302004
3Department of Chemistry and College, University of Delhi, India, 110019
4Department of Chemistry, Lingayas University, Faridabad, Haryana, India 121002
5Department of Chemistry, Ganpat University, Mehsana, Gujarat, India 384012
6Department of Chemistry, University of Kota, Kota, Rajasthan, India 324005
Corresponding Author Email: 1988rajeshmeena@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170206
Article Publishing History
Article Received on : 31-July-2020
Article Accepted on : 26-Aug-2020
Article Published : 29 Aug 2020
Plagiarism Check: Yes
Reviewed by: Manoj Banjare
Second Review by: Fageria Pragati
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
The silver nanoparticle was successfully synthesized by using the help of Phyllanthus emblica plant extract as a reducing agent and aqueous silver nitrate as the precursor. Moreover, physical and chemical methods are widely used for the synthesis of nanoparticles, but these methods have expensive and not ecofriendly. This study highlights the green, rapid, facile, cost-effective, and ecofriendly synthesis and synthesized nanoparticles also investigate their antibacterial activity. Synthesized silver nanoparticles are analyzed by different techniques of modes like XRD, UV-Visible spectroscopy, TEM, FTIR, and photoluminescence (PL). The prepared AgNPs show characteristic absorption peak in UV-Visible spectroscopy due to SPR (surface plasmonic resonance) band between 400 to 450 nm wavelength, which was confirmed by TEM (transmission electron microscopy) image. X-ray diffraction (XRD) results showed the crystalline nature of AgNPs as well as the size of nanoparticles calculated with the help of TEM (20-25 nm) and XRD (25 nm). ATR spectroscopy identified the functional groups that are involved in the reduction of silver ion to AgNPs and the PL spectrum indicates higher emission in the green region and low emission peak in the UV region. Antibacterial activity of AgNPs analyzed against with the help of E.Coli bacteria and the result shows that a higher concentration of AgNPs is increasing as well as a zone of inhibition increased. This method is environmentally friendly, of low cost, and less expensive method for the fabrication of AgNPs in abundance which can be further helpful for biosensor devices as well as for other applications such as pollutant degradation, pharmaceutical, and hydrogen production, etc therefore can promote the application of green technology for the production of AgNPs.
KEYWORDS:
Antibacterial Activity; AgNPs; FTIR; Photoluminescence; Surface Plasmon Resonance; TEM; UV-Vis; XRD
Copy the following to cite this article:
Meena R. K, Meena R, Arya D. K, Jadoun S, Hada R, Kumari R. Synthesis of Silver Nanoparticles by Phyllanthus emblica Plant Extract and Their Antibacterial Activity. Mat. Sci. Res. India; Special Issue (2020).
|
Copy the following to cite this URL:
Meena R. K, Meena R, Arya D. K, Jadoun S, Hada R, Kumari R. Synthesis of Silver Nanoparticles by Phyllanthus emblica Plant Extract and Their Antibacterial Activity. Mat. Sci. Res. India; Special Issue (2020). Available from: https://bit.ly/3hK1NGf
|
Introduction
The specific characteristics features of metal nanoparticles participate in the field of energy, optics along with biomedicine as well as disaster management with other health care issues.1 Among the various nanoparticles like Ag, Au, Pt, and Pd, etc. are their broad range of applications like antibacterial agent, photocatalyst in a photocatalytic reaction as well as a biosensor technology. Generally, researches are prepared of silver nanoparticles by a number of the process such as chemical reduction,2 electrochemical reduction and photochemical reduction3 but such type of convention method requires more amount of chemicals as well as a large amount of energy for nanomaterial synthesis process so such type of synthesized products are more hazardous for the environment.4 Different types physical and chemical methods apply for preparation of silver nanoparticles for various shape and size of nanoparticles, and such type of technique are involved in UV irradiation5,6 microwave irradiation7,8 chemical reduction9-11 photochemical method12,13 electron irradiation14,15 and sonoelectrochemical method.16 However extremely all previous methods in engaging more than one step as well as advanced energy, low conversions, the trouble of purification, and required critical chemicals. All chemical synthesis methods of nanomaterial show the poisonous chemicals, but the synthesis of nanomaterial by the green method can be removed from this issue, so it requires developing a green synthesis method for nanomaterial synthesis. A wide range of metal NPs such as Ag,17-18 Au,19-23 ZnO,24 TiO2,25 CuO,26 In2O3,27 etc, have been synthesized using the help of herbal plant extracts. Various, plants have been used for the synthesis of metal nanoparticles including medicinal plants. Ag has long been reputed for its goods inhibitory effect on many bacterial strains and catalytic activity. So, normally used in industrial and medical products so, in this research work, we have synthesized AgNPs with the help of Phyllanthus emblica plant extract for the conversion of silver ion to silver nanoparticles by silver nitrate solution.
Present investigation focused on the effects of the different optical parameters on the silver nanoparticles and investigates of the antibacterial effect of the synthesized AgNPs. Phyllanthus emblica is a common plant, which is more amount of exceedingly in a nearby part of Indian subcontinents as shown in figure 1. Some organic phytochemicals compounds are present in plant extract so Phyllanthus emblica fruit extract can be used in the synthesis of another different type of nanoparticles like Au, Ag, Pd, Pt, ZnO, and TiO2, etc. Present phytochemicals in plant extract such as terpenoids and flavanones which act as stabilizing and reducing agents for the nanoparticle synthesis process. Synthesized AgNPs show the no harmful effects on the human body as well as environmental health, natural antibacterial activity in the direction of pathogens like as viruses, fungi and bacteria etc.
Experimental
Phyllanthus emblica Extract
Sigma-Aldrich grade AgNO3 (99%) solutions was prepared by distilled water and all glassware was purified by distilled water and dry and glassware was autoclaved before the experiment. Extraction of plant fruit and ready the aqueous plant extract28 by the dry fruit. Firstly washed carefully by distilled water to make free from dust particles and dried 24 h on room temperature. After that 2 gm dry fruit was soaked in 50 mL of pure distilled water for 24 h. Then synthesized plant extract was filtered and stored for nanoparticle synthesis.
Nanoparticle Synthesis
Synthesis of AgNPs by green synthesis process.29 Phyllanthus emblica fruit extract (0.5% (w/v)) was mixed drop by drop in aqueous 0.001 M AgNO3 solution and kept in boiling tube at around 25oC temperature till 12 h aging. Then the solution of a mixture of silver nitrate turns brown with increasing time interval; its optical property shows the confirmation of AgNPs. Synthesized silver nanoparticles are established without any chemical reagent and their stability for a long time due to antimicrobial properties of this plant extract and now synthesized silver nanoparticles was separated by centrifugation technique at 5000 rpm for 20 minutes. For the further settlement of particles, the supernatant material was transferred to a beaker and frequent centrifugation process was carried out to clean AgNPs. The obtained nanoparticle pellet was dry in an oven and stored for further study.
Results and Discussion
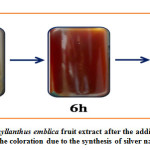
Synthesis of AgNPs was noted by optical observation with reaction time, initially a silver salt solution is mixed to Phyllanthus emblica plant extract, it observed a color change from light yellow to brown color within 12 h of incubation as shown figure 2, after that the color of the solution not be changed and stable therefore observed change of color is strong indication formation of nanoparticles and the stability of color shown that means it’s have been completely done the conversion of silver ions to silver nanoparticle due to SPR. 30-33
It’s indicated these plants extract reducing agents such as terpenoids and flavanones are responsible for the reduction of Ag+ to AgNPs. It is suggested, the time duration is an important role during the nanoparticle synthesis process, and present analysis indicates that’s 12 h time duration is more appropriate for the complete synthesis of silver nanoparticles.
UV-Visible Analysis
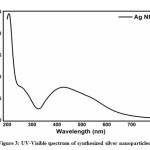
Synthesized AgNPs were characterized and confirmed by respective UV-visible absorption band at appearing around 400-450 nm at 12 h shown in figure 3, due to the strong surface plasmon resonance. This wavelength corresponds to the AgNPs and a previous study revealed that the SPR value for the AgNPs was in the range 400- 450 nm [34-36]. After 12 h of reaction time UV-visible absorption band did not change peak position and highly stable which means 12 h reaction period is more reliable for synthesis and stability of silver nanoparticles.
Surface plasmon resonance (SPR) is the excitation of free electrons within the conduction band. Hence the broad absorbance was observed. This broad absorbance, indicating the amount of synthesized AgNPs. The broad spectra could be generated due to some reasons (1) organic elements that are produced by plant extract (2) homogeneity,37-38 and extra-fine nature of AgNPs39 (3) Moreover many other factors such as the size and shape of nanoparticles.40 The TEM image from this study confirms the spherical shape of synthesized silver nanoparticles.
XRD Analysis
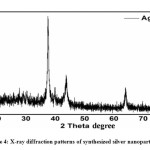
Figure. 4 shows the XRD patterns of synthesized AgNPs by Phyllanthus emblica extract. Some intense characteristic diffraction peaks at 2θ angles of 38.4, 44.5, 64.8, 77.6 are observed, which crystallographic planes of (111), (200), (220), and (311). According to the TEM, synthesized AgNPs are evidently showed the most AgNPs were highly dispersed in spherical shapes. Synthesized silver nanoparticle directly corresponds with JCPDS 00-004-0783. No, any other peaks were obtained, only cubic shape AgNPs appeared. Usually, XRD peaks width directly related to the crystallite size of nanomaterial. Which are useful in the debye scherrer equation for the analysis of average particle size.41 That means Diffraction peak D = (kλ)/(β cos θ), here λ wavelength of Cukα, D is the crystalline size of the sample powder, β is the full width at half maxima, θ is the bragg diffraction angle and the K is the constant. The highest peak (111) was picked to determine the crystalline size of nanomaterial. According to this equation, the average size of AgNPs is found 25 nm and mostly spherical. That’s the result that was found consistent with the TEM analysis.
TEM Analysis
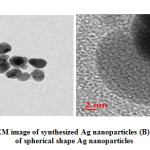
Synthesized silver nanoparticles shape and size are calculated by with the help of HRTEM image shows in figure 5. Aliquots silver nanoparticle solutions were placed on a copper grid and dry under ambient conditions than after the HRTEM image was recorded. These results shows the particles are still in the range of nm scale with a spherical shape. These spherical shape synthesized AgNPs with a size range of 20-25 nm.
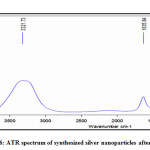
ATR Analysis
ATR measurement was used for identifying the responsible biomolecules for capping and stabilizing agent in AgNPs. Two types of infrared bands are observed at a position of 3321cm-1 and 1635 cm-1 figure 6. Present intense peak around at 3321 cm-1 responsible for OH stretching of alcoholic and phenolic groups42 and another intense peak around at 1635 cm-1 appear from the C=O stretching mode of amine group these groups are found in proteins43 and these groups of proteins as a capping and stabilizing agent for synthesized AgNPs, which are responsible for increasing the stability of the synthesized nanoparticles.44 It is observed the structures of proteins were not affected by the silver ions before and after binding with AgNPs.45 Previous studies by 46-48 have also identified the plant extracts in the samples and proposed that these alcoholic and phenolic groups could serve as organic reducing as well as capping agents.
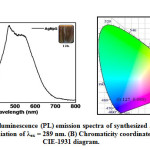
Photoluminescence Analysis
Figure. 7 represents the photoluminescence spectra of the synthesized silver nanoparticles by Phyllanthus emblica plant extract that are studied through emission spectroscopy. This is one of the technical methods for observation of the optical properties of synthesized AgNPs. Synthesized AgNPs are mixed in distilled water then emission spectra are obtained within a range from 200 to 800 and for the wavelength of excitation at 289 nm. It exhibits the emission peaks around 400 to 600 nm which belong to the green-yellow region. The intensity of emission peak continues increased up to 469 nm, then after it’s gradually decreased up to 650 nm. The luminescence spectrum of AgNPs is generated by the excitation of an electron from occupied d bands into states above the Fermi levels its responsible for luminescence spectra of AgNPs [49] and broad peak in green-yellow emission is produced by radial recombination of photoexcited holes with the electrons in oxygen vacancies and indicates that AgNPs have a good crystal structure.
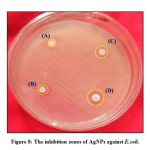
Antibacterial Activities
Antibacterial activity of synthesized silver nanoparticles analysed against E.Coli bacterial samples, shown in figure.8. During this process in which synthesized silver nanoparticles are used in different concentrations (2, 4, 8, and 10mg) are further agar dishes containing colony. The dishes are visible every concentration with a bacterial colony and experimentally observed after 24 h incubating time at 37oC. The zone of approval is experiential maximum at 10 mg of AgNPs. The antibacterial activity results show due to modifying bacteria cell permeability as well as enzyme degradation by synthesized AgNPs. The resultant zone of inhibition increased as well as increases the concentration of AgNPs.
Conclusions
We have reported a reasonable, eco-friendly, and easy approach for the synthesis of AgNPs. Phyllanthus emblica plant extracts which act as a reducing, stabilizing, and capping agent for nanoparticle synthesis. This is a simple, green efficient method for the synthesis of silver nanoparticles at room temperature without using any harmful reducing and capping agent. The green synthesized silver nanoparticles were collected of spherical shape which was highly stable and crystalline. These AgNPs were characterized carefully by XRD, TEM, FTIR, PL, and UV-visible spectroscopy. The UV-visible spectra showed a characteristic range of 410-425 nm for nanoparticle. XRD results showed the crystalline nature of AgNPs, and size with the help of TEM (20-25 nm) and XRD (25 nm). ATR analysis found numerous phytochemicals and functional groups these groups are responsible for stabilizing and creation nanoparticle. Antibacterial activity of AgNPs analyzed against E.Coli bacteria and the result shows that a higher concentration of AgNPs is increasing as well as the zone of inhibition increased. This synthesizes process is cheaper, a single step, and faster as compared to chemical and biological methods as well as eco friendly, of low cost, and simple therefore can promote the application of the green method for silver nanoparticle synthesis.
Acknowledgement
All authors provided critical feedback and helped shape the research, analysis and manuscript as well as we gratefully acknowledge, Department of Botany, Rajasthan University, Jaipur for the biological activity measurement of our samples and the Materials Research Centre MNIT Jaipur, and Department of Chemistry, Kalindi College, University of Delhi, for providing help in characterization and synthesis of samples.
Funding Source
This research received no specific grant from any funding agency.
Conflict of Interest
There is no conflict of interests regarding the publication of this article.
References
- A Fayaz, M Balaji, M Girilal, R Yadav, P Thangavelu, KR Venketesan. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics. A study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology and Medicine. 6:103-109 (2010).
CrossRef
- K Balantrapu, D Goia. Silver nanoparticles for printable electronics and biological applications. Journal of Materials Research. 24:2828-2836 (2009).
CrossRef
- RK Bera, AK Das, CR Raj. Fabrication of Carbon Nanotube/Indium Tin Oxide “Inverse Tandem” Absorbing Coatings with Tunable Spectral Selectivity for Solar-Thermal Applications. Chemistry of Materials. 4505-4511 (2010).
- AM Awwad, NM Salem. Green synthesis of silver nano- particles by mulberry leaves extract. Journal of Nanoscience and Nanotechnology. 2:125-128 (2011).
CrossRef
- Le AT, Tam LT, Tam PD, et al. Synthesis of oleic acid-stabilized silver nanoparticles and analysis of their antibacterial activity. Materials Science and Engineering: C. 30:910-916 (2010).
CrossRef
- H Huang, Y Yang. Preparation of silver nanoparticles in inorganic clay suspensions. Composites Science and Technology. 68:2948-2953 (2008).
CrossRef
- H Yin, T Yamamoto, Y Wada, S Yanagida. Large-scale and size-controlled synthesis of silver nanoparticles under microwave irradiation. Materials Chemistry and Physics. 83:66-70 (2004).
CrossRef
- MN Nadagouda, TF Speth, RS Varma. Microwave-Assisted Green Synthesis of Silver Nanostructures. Accounts of Chemical Research. 44:469-478 (2011).
CrossRef
- L Suber, I Sondi, E Matijevic, DV Goia. Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions. Journal of Colloid and Interface Science. 288:489-495 (2005).
CrossRef
- K Song, S Lee, T Park, B Lee. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean Journal of Chemical Engineering. 26:153-155 (2009).
CrossRef
- O.Yu. Golubeva, O.V. Shamova, D Orlov, T Pazina, A.S. Boldina, V.N. Kokryakov. Study of antimicrobial and hemolytic activities of silver nanoparticles prepared by chemical reduction. Glass Physics and Chemistry. 36:628-634 (2010).
CrossRef
- M Harada, C Kawasaki, K Saijo, M Demizu, Y Kimura. Photochemical synthesis of silver particles using water-in-ionic liquid microemulsions in high-pressure CO2. Journal of Colloid and Interface Science. 343:537-545 (2010).
CrossRef
- M Harada, Y Kimura, K Saijo, T Ogawa, S Isoda. Photochemical synthesis of silver particles in Tween 20/water/ionic liquid microemulsions. Journal of Colloid and Interface Science. 339:373-381 (2009).
CrossRef
- K Li, FS Zhang. A novel approach for preparing silver nanoparticles under electron beam irradiation. Journal of Nanoparticle Research. 12:1423-1428 (2010).
CrossRef
- KA Bogle, et al. Silver nanoparticles: synthesis and size control by electron irradiation. Nanotechnology. 17:3204 (2006).
CrossRef
- J Zhu, S Liu, O Palchik, Y Koltypin, A Gedanken. Shape-Controlled Synthesis of Silver Nanoparticles by Pulse Sonoelectrochemical Methods. Langmuir. 16:6396-6399 (2000).
CrossRef
- R.P. Singh, S. Magesh, C. Rakkiyappan. Formation of fenugreek (Trigonella foenumgraecum) extract mediated Ag nanoparticles: mechanism and applications. International Journal of Bio-Science and Bio-Technology. 02:75-80 (2011).
- K. Govindaraju, S. Tamilselvan, V. Kiruthiga, G. Singaravelu. Biogenic silver nanoparticles by Solanum torvum and their promising antimicrobial activity. Journal of Biopesticides. 3:394-399 (2010).
- E.M. Egorova, A.A. Revina. Synthesis of metallic nanoparticles in reverse micelles in the presence of quercetin. Colloids Surfaces. Colloids and Surfaces A: Physicochemical and Engineering. 168:87-96 (2000).
CrossRef
- J. Huang, Q. Li, D. Sun, Y. Lu, X. Yang, H. Wang, Y. Wang, W. Shao, N. He, J. Hong, C. Chen. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 8:105104 (2007).
CrossRef
- B. Ankamwar, C. Damle, A. Ahmad, M. Sastry. Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. Journal of Nanoscience and Nanotechnology. 5:1665-1671 (2005).
CrossRef
- S.P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad, M. Sastry. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnology Progress. 22:577-583 (2006).
CrossRef
- S.S. Shankar, A. Rai, A. Ahmad, M. Sastry. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of Colloid and Interface Science. Sci. 275:496-502 (2004).
CrossRef
- H.M. Gong, L. Zhou, X.R. Su, S. Xiao, S.D. Liu, Q.Q.Wang. H. M. Gong, L. Zhou, X. R. Su, S. Xiao, S. D. Liu, and Q. Q. Wang. Illuminating dark plasmons of silver nanoantenna rings to enhance exciton–plasmon interactions. Advanced Functional Materials.19:298-303 (2009).
CrossRef
- M. Sundarajan, S. Gowri. Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Letters. 8:447-451 (2011).
- N.P.S. Acharyulu, R.S. Dubey, V. Swaminadham, P. Kollu, S.V.N. Pammi. Green Synthesis of CuO Nanoparticles using Phyllanthus Amarus Leaf Extract and theirAntibacterial Activity Against Multidrug Resistance Bacteria. International Journal of Engineering Research and Technology. 4:639-641 (2014).
- S. Maensiri, P. Laokul, J. Klinkaewnarong, S. Phokha, V. Promarak, S. Seraphin, Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: synthesis and optical properties. Journal of Optoelectronics and Advanced Materials. 10:161-165 (2008).
- R. Manikandan, M Beulaja, R. Thiagarajan, S. Palanisamy, G. Goutham, A. Koodalingam. Biosynthesis of silver nanoparticles using aqueous extract of Phyllanthus acidus L. fruits and characterization of its anti-inflammatory effect against H2O2 exposed rat peritoneal macrophages. Process Biochemistry. 55:172-181 (2017).
CrossRef
- J Virkutyte, RS Varma, J Virkutyte, RS Varma. Fabrication and visible-light photocatalytic activity of novel Ag/TiO2-xNx photocatalyst. New Journal of Chemistry. 34:1094-1096 (2010).
CrossRef
- MJ Kwon, J Lee, AW Wark, HJ Lee. Nanoparticles-enhanced surface plasmon resonance detection of proteins at attomolar concentrations: comparing different nanoparticle shape and size. Analytical Chemistry. 84:1702-1707 (2012).
CrossRef
- PC Ray. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chemical Reviews. 110:5332-5365 (2010).
CrossRef
- V Amendola, OM Bakr, F stellacci. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: effect of shape, size, structure and assembly. Plasmonics. 5:85-97 (2010).
CrossRef
- GV Hartland. Optical studies of dynamic in noble metal nanostructures. Chemical Reviews. 111:3858-3887 (2011).
CrossRef
- S. Maiti, G. Barman and J. K. Laha, Synthesis of silver nanoparticles having different morphologies and its application in estimation of chlorpyrifos. Advanced Science Focus. 1:145-150 (2013).
CrossRef
- S. Maiti, D. Krishnan, G. Barman, S. K. Ghosh and J. K. Laha, Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon eslantum extract. Journal of Analytical Science and Technology. 5:40-46 (2014).
CrossRef
- Z. Zaheer and Rafiuddin, Silver nanoparticles to self-assembled films: green synthesis and characterization. Colloids and Surfaces B: Biointerfaces. 90:48-52 (2012).
CrossRef
- K. Shameli, M. B. Ahmad, S. D. Jazayeri et al. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. International Journal of Molecular Science. 13:6639-6650 (2012).
CrossRef
- M. Zargar, A. A. Hamid, F. A. Bakar et al. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 16:6667-6676 (2011).
CrossRef
- K. Shameli, M. B. Ahmad, E. A. Jaffar Al-Mulla et al. Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction. Molecules. 17:8506-8517 (2012).
CrossRef
- A. T. M. Saeb, A. S. Alshammari, H. Al-Brahim, and K. A. Al-Rubeaan, Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. The Scientific World Journal. 9 pages, (2014).
CrossRef
- B Ajitha, Ashok Kumar Reddy, P Sreedhara Reddy. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim Acta Part A. 121:164-172 (2014).
CrossRef
- T. Wriedt, W. Hergert. Mie Theory. Universität Bremen, Bremen. 17-21 (2008).
- H Jiale, L Qingbiao, S Daohua. Et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 18:105104 (2007).
CrossRef
- G Varsanyi, Assignments of Vibrational Spectra of Seven Hundred Benzene Derivatives. Halsted Press vol. 1 (1974).
- I.D.G Macdonald, W.E Smith. Orientation of Cytochrome c Adsorbed on a Citrate-Reduced Silver Colloid Surface. Langmuir. 12:706-713 (1996).
CrossRef
- D. Philip, Mangifera Indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 78:327-331 (2011).
CrossRef
- D. Philip, Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E: Low-Dimensional Systems and Nanostructures. 42:1417–1424 (2010).
CrossRef
- P. Velmurugan, S. Lee, M. Iydroose, K. Lee, and B. Oh. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Applied Microbiology and Biotechnology. 97:361-368 (2013).
CrossRef
- B.M.V Romão, C Pardini, R.C.L Dutra, F Burel. Caracterização por FT-IR de Agentes de Cura Utilizados em Resinas Epoxídicas-II-Polimercaptana, Poliaminoamida e Amina Modificada. Polímeros Ciência Tecnologia. 13:173-180 (2003).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Risikesh Meena2
, Risikesh Meena2 , Dinesh Kumar Arya3
, Dinesh Kumar Arya3 , Sapana Jadoun4
, Sapana Jadoun4 , Renu Hada5
, Renu Hada5 and Roopa Kumari6
and Roopa Kumari6
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal