Alaa Fehaid1 , Sara T. Elazab2, Mona G. Elhadidy3
, Sara T. Elazab2, Mona G. Elhadidy3 and Mahmoud M. Elalfy1*
and Mahmoud M. Elalfy1*
1Forensic Medicine and Toxicology Department, Faculty of Veterinary Medicine, Mansoura University, Egypt.
2Department of Pharmacology, Faculty of Veterinary Medicine, Mansoura University, Egypt.
3Medical Physiology, Faculty of Medicine, Mansoura University, Egypt, and Physiology Department, Faculty of Medicine, Al Baha University, KSA.
Corresponding Author E-mail: mahmoudelalfy@mans.edu.eg
DOI : http://dx.doi.org/10.13005/msri/190102
Article Publishing History
Article Received on : 22 Mar 22
Article Accepted on : 19 Apr 22
Article Published : 25 Apr 2022
Plagiarism Check: Yes
Reviewed by: Raana Sarvari Second Review by: Dr. Mehdihasan Ijubhai
Second Review by: Dr. Mehdihasan Ijubhai  Final Approval by: Dr. Jaymin Ray
Final Approval by: Dr. Jaymin Ray
Article Metrics
ABSTRACT:
Silver nanoparticles have been shown to increase postnatal toxicity in breastfeeding female rats, with negative consequences for their offspring. We wanted to investigate more about the differences in toxicity between silver nitrate and silver nanoparticles, as well as the impact of zinc chloride treatment on the silver nitrate induced toxicity on female albino rats. For 21 days, 30 breastfeeding female albino rats were divided into 4 groups; the first three group received orally 0, 50,100 mg/kg of silver nitrate. While the fourth group received 100 mg/kg of silver nitrate plus 300 ppm of zinc chloride in drinking water and fifth group received only AG-NPs at dose of 100 ppm. The results demonstrated that silver nitrate were more hazardous than nano-silver, as evidenced by higher free radical release, increased MDA levels, and decreased antioxidant enzyme levels (SOD). In addition, the silver ions-treated group had higher levels of liver enzymes and creatinine.
KEYWORDS:
Hepatorenal Functions; MDA; Silver Nitrates; Silver Ions; sod1
Copy the following to cite this article:
Fehaid A. Elazab S. T, Elhadidy M. G, M. A, Elalfy. M. M. Zinc Chloride Ameliorates the Adverse Effects of Silver Nitrates Compared o Silver Nanoparticle In Post-Natal Model Of Toxicity.Mat. Sci. Res. India;19(1).
|
Copy the following to cite this URL:
Fehaid A. Elazab S. T, Elhadidy M. G, M. A, Elalfy. M. M. Zinc Chloride Ameliorates the Adverse Effects of Silver Nitrates Compared o Silver Nanoparticle In Post-Natal Model Of Toxicity.Mat. Sci. Res. India;19(1). Available from: https://bit.ly/3LdxN4n
|
Introduction
Since the early centuries, silver ions have been used as a biocide, antibacterial, disinfectant, and antiseptic against pandemic diseases such as cholera, eye infection, and burn wounds 1. Silver-based products have recently been employed in a variety of food industries, including food packaging materials, medical healthcare, and hard surface materials and textiles.2, 3.
There is a lot of evidence that silver nitrates or nano-silver are present in the food chain. 4 and its toxicity to animals and humans could be increased as residues and in the ecology around us. The yearly release of silver into the ecosystem through industrial wastes and emissions was estimated to be around 2,500 tonnes, with 80 tonnes contaminating surface waters. 5, 6. Silver ions could be released into the environment as a result of silver nitrate pollution or silver nanoparticles. 4. Silver ion residues have been found in milk, meat, and water sources. 6, 7.
In a prior post-natal toxicity model, the hepatorenal toxicity of silver nanoparticles in feti development and dams of rats was documented. 8 and according to many research, silver nanoparticles caused reproductive, respiratory, and cutaneous toxicity. 9, 10.
More than 300 enzymes need zinc as a cofactor in the majority of metabolic processes and a wide range of cellular processes 11. In addition, zinc supplementation improved the effects of nanosilver on brain cells 12 and the use of hazardous dosages of silver nitrates resulted in a decrease in intracellular zinc and iron levels 13.
Notably, in rats, zinc had a protective effect against silver induced toxicity 14. Similarly, zinc increased the antioxidant state of nickel treated rats’ livers 15. Also, zinc protected the liver and kidneys from mercury induced injuries 16, 17. Zinc level is critical for tissue regeneration following tissue injury because zinc regulates cytokine expression, suppresses inflammation, and is essential to activate antioxidant enzymes that scavenge reactive oxygen species and lowering oxidative stress 18.
With a special comparison to silver nanoparticles, this
study postulated that zinc chloride would create a protective effect against
silver nitrate-induced toxicity in breastfeeding female rats and their puppies .
Materials and Methods
Laboratory animals
Female albino rats weighing
180 to 200 grammes were purchased from the experimental section of Mansoura
University’s Faculty of Pharmacy. The animals appeared to be in good health and
were kept in plastic cages with wood shavings as bedding. All animals were kept
in plastic cages in groups of four in a regulated environment (25 3 °C
temperature and 45–65 percent humidity) and in illuminated rooms with a 12-hour
light/dark cycle. Animals were housed for two weeks prior to the experiment and
fed a balanced ration with free access to food and water. We observe all animal
ethics rules at the college of veterinary medicine in Mansoura, as well as all
experiments conducted in the forensic medicine and toxicology department of the
faculty of veterinary medicine in Mansoura, Egypt, under code number (R/68).
Tested chemicals
Silver nitrates and zinc chloride
were purshased from Sigma Aldrich ( USA).
Experimental grouping and design
Thirty pregnant female rats divided into three groups each one contained six rats weighed 150 to 200 g and kept until delivery. In the current study, Silver nitrates (Sigma Aldrich, USA) given orally, dissolved in di-ionized water, at dose level 0, 50, 100 ppm (equivalent to 1/50 and 1/100 of the LD50 recorded earlier on first day of lactation till 21 days and control group intubated with di-ionized water as control. The fourth group received daily 100 ppm of silver nitrates plus zinc chloride as water supplement at dose of 300 ppm/L. Dams were weighted prior to dosing to provide a consistent dose throughout the trial. On the 21st day of lactation, all rats were slaughtered with an overdose of thiopental, blood was collected from dams and puppies for serum separation, and different tissue samples such as liver, kidney, and lung were taken. Puppies’ liver, kidney, and lung tissues were isolated and fixed in buffered formalin for histological analysis. The bodies of the other euthanized rats were discarded using the technique outlined before19. For comparison with silver ion, we also give data from a previously published article on nano-silver treatments of postnatal models at a dose of 1/50 of LD50 equivalent to 100 mg/kg (group 5).8.
Histopathological Examination
Different tissues from dams and puppies were fixed in 10% neutral buffered formalin, including liver, kidney, and lung. For pathology detection, fixed tissue was stained with traditional eosin and hematoxylin staining, imaging, and reading. 20.
Biochemical analysis
Sera from all dams and puppies in the treated and control groups were
examined for AST, ALT, cholesterol, BUN, and creatinine levels to assess the
postnatal toxicity of silver nitrates and compare it to zinc therapies.
Oxidant/antioxidants index
To investigate the toxicity of silver ions and the
protection of zinc by kits, we evaluated the oxidant/antioxidant MDA (oxidant),
sod1, and GSH (antioxidants) in dams’ liver tissue homogenates.
Statistical Analysis
For homogeneity, sample size and statistical difference between groups, all experimental data was evaluated using One-way Annova and the turkey test.. P<0.05 was considered significant (SPSS version 20).
Results
When compared to the control group, silver nitrates had no significant effects on body weight. Furthermore, silver nitrates caused hepatomegaly, particularly at higher doses of 100 mg/kg, while zinc kept the same liver weight as the control group. Furthermore, the weight of the liver was unaffected by silver nitrates. In particular, when compared to the control group, oral treatment of silver nitrate at a dose of 100 mg/kg resulted in significant increases in liver enzyme, urea, creatinine, and cholesterol levels in dams and puppies . While zinc treatment at a dose of 300 mg/L restored hepatorenal parameters to their baseline levels, hepatorenal parameters remained identical to control. At a dose of 100 mg/kg, silver nitrate was found to be more hazardous than AG-NPs. (see table 1, table 2).
Table 1: In postnatal toxicity in rat dams, oral administration of silver nitrate treatment had hepatorenal consequences.
|
|
ALT
|
AST
|
UREA
|
Creatinine
|
cholesterol
|
|
Group1 Control
|
9.3937±1.88508
|
40.1906± 5.977
|
40.8418±1.9
|
0.3333± 0.04
|
23.3333± 4.3
|
|
Group 2
|
14.86 ±0.9184
|
42.0167±0.95
|
44.0633± 1.8
|
0.5123±0.003
|
55.6667±4.9
|
|
Group 3 (ttt name should be mentioned)
|
25.6±0.55076
|
74.3933± 8.2
|
101.31±1.5
|
0.855±0.04
|
103.31±6.7
|
|
Group 4
|
9.1660. ±. 0.33
|
40 ±.0.57735
|
40.333± 0.33
|
0.3547±0.05
|
23.87± 4.7
|
|
Group 5
(AG-NPS)
|
24.73889±0.9
|
45.29567±0.04
|
45.65889±0.3
|
0.755±0.04
|
153.9027±0.3.1
|
Group 1 served as control, Group2
received 1/20 of LD50 of silver nitrates (equivalent to 50 mg/kg) , group 3
received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg), group 4
received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg) + 300 mg of
zinc chloride and group 5 received 1/10 of silver nanoparticle (equivalent to
100 mg/kg). p value at ≤ 0.05 considered significant between groups.
Table 2 shows that silver
nitrates treatments increased liver toxicity as seen by higher levels of ALT
and AST, as well as produced renal damage as evidenced by increased levels of
creatinine and urea, particularly in the feti of rat dams given a dose of 100
mg/kg silver nitrates. Zinc chloride improved the hepatorenal functions of rat
dams treated with silver nitrates at a level of 100 mg/kg. Furthermore,
nano-silver had a lower harmful effect in feti than silver nitrates, as
evidenced by lower levels of ALT, urea, creatinine, and cholesterol in
nano-silver treated dams compared to silver nitrates treated dams, with the
exception of the level of AST.
Table 2: Silver nitrates have hepatorenal consequences in rat puppies, as demonstrated by serum biochemical markers.
|
|
ALT
|
AST
|
UREA
|
Creatinine
|
cholesterol
|
|
Group 1 Control
|
7.2010±1.03
|
44.2329±3.3
|
33.7310±2.4
|
0.2433±0.01
|
29.6667±4.3
|
|
Group 2
|
10.3433±1.04
|
36.3333±1.8
|
76.5610±2.9
|
0.3563±0.02
|
45.8733±3.7
|
|
Group 3
|
19.7800±1.03
|
50.0867±3
|
119.5553±6.1
|
0.5957±0.09
|
62.0500±2.2
|
|
Group 4
|
7.2333±0.08
|
33.3333±1.2
|
36.3333±2.02
|
0.2467±0.08
|
29.6667±4.8
|
|
Group 5
(AG-NPS)
|
16.72±1.83a
|
81.13±7.37a
|
80.27±6.48ab
|
0.3849±1.86
|
11.64±0.73a
|
Group 1 served as control, Group2
received 1/20 of LD50 of silver nitrates (equivalent to 50 mg/kg), group 3
received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg), group 4
received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg) + 300 mg of
zinc chloride and group 5 received 1/10 of silver nanoparticle (equivalent to
100 mg/kg). p value at ≤ 0.05 considered significant between groups.
Table 3 shows that silver
nitrates increased oxidant as MDA in a dose-dependent manner as compared to the
control group and dramatically reduced GSH and SOD1 levels. Silver
nanoparticles were shown to be less hazardous than silver ions, with lower MDA
induction and lower GSH and SOD1 reductions than silver nitrate-treated groups.
MDA, SOD1, and GSH levels in the zinc chloride group were similar to those in
the control group.
Table 3: Oxidant/antioxidant effects of silver nitrates on liver homogenates of dams.
|
Groups
|
MDA
|
GSH
|
SOD1
|
|
Group 1
|
614.1171±32.70247
|
6.5143±0.32617
|
399.7194±35.7
|
|
Group 2
|
712.6250±13.45752
|
5.6625±0.29395
|
355.0000±30.7
|
|
Group 3
|
800.5000±33.46925
|
4.8750±0.12500
|
286.3750±28
|
|
Gropup4
|
614.0764±32.73509
|
6.4845±0.37571
|
399.7194±35
|
|
Group 5
(AG-NPS)
|
625.2097±31.707
|
6.796657±0.125
|
407.0506±35.2
|
Group 1 served as control, Group2 received 1/20 of LD50 of silver nitrates (equivalent to 50 mg/kg), group 3 received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg), group 4 received 1/10 of LD50 of silver nitrates (equivalent to 100 mg/kg) + 300 mg of zinc chloride and group 5 received 1/10 of silver nanoparticle (equivalent to 100 mg/kg). p-value at ≤ 0.05 considered significant between groups.
Pathological findings
Hemorrhage, necrosis, and leucocytic infiltration in the liver, alveolar atelectasis, and renal degeneration were all caused by silver nitrates. Furthermore, silver nitrates were more harmful than nano-silver since their nanoparticle only caused intralobular fibroblastic growth in the liver, bleeding in the lungs, glomerular capillary congestion, and renal glomeruli proliferation. In addition, zinc therapies reduced the negative effects of silver nitrates on liver and kidney tissue but did not restore lung tissue to its original state (fig 2). Moreover, feti of dams received silver nitrates displayed a signet ring appearance of the hepatocytes where sharp clear vacuoles pushing nucleus of hepatocytes to the periphery, kidney showed a proliferation of the mesangial cells in the renal glomeruli and degenerative changes in the renal tubular epithelium lining renal tubules and lung shown an atelectasis of pulmonary alveoli and compensatory emphysema in neighboring alveoli (fig 3 a-d). While the feti of dams treated with silver nanoparticles showed a local necrosis of hepatocytes, the kidney of a suckling feti (21 day old age) from dam received 100 mg/kg (1/50 LD50) AG-NPs from delivery until weaning, showing a congestion of interstitial blood capillaries, and the lung of a suckling feti (21 day old age) from dam received 100 mg/kg (1/50 LD50 (fig 3 d-f)
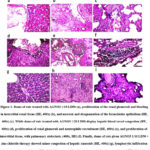 |
Figure 1: Dams of rats treated with AGNO3 1/10 LD50 (a), proliferation of the renal glomeruli and bleeding in interstitial renal tissue (HE, 400x) (b), and necrosis and desquamation of the bronchiolar epithelium (HE, 400x) (c).
Click here to View figure
|
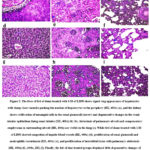 |
Figure 2: The liver of feti of dams treated with 1/10 of LD50 shows signet ring appearance of hepatocytes with sharp clear vacuoles pushing the nucleus of hepatocytes to the periphery (HE, 400x).
Click here to View figure
|
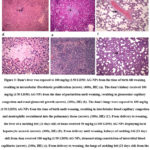 |
Figure 3: Dam’s liver was exposed to 100 mg/kg (1/50 LD50) AG-NPs from the time of birth till weaning, resulting in intralobular fibroblastic proliferation (arrow).
Click here to View figure
|
Discussion
Toxicological studies on silver nitrates and its nanoparticle on animal, human, and environmental health have increased because of the increased usage of silver nanoparticle in animal feed and human medicinal resources 21. We previously reported on silver nanoparticles’ maternal toxicity in lactating female albino rats and their puppies 8. When compared to the control group, silver nitrates or nano-silver treatments had no influence on body weight, liver to body weight ratio, or puppies after 21 days. Similarly, after 28 days of repeated oral administration, both silver nitrates and its nano form had no influence on the body weights of male and female rats, according to Qin et al. 21 and kim et al. 22
When compared to the control group, silver nitrates’ cytotoxicity was more evident than its nano-form in nursing female albino rats after 21 days of treatment. This toxicity was revealed by an increase in liver enzymes, creatinine, blood urea nitrogen, cholesterol, and a decrease in total protein and albumin levels, despite the fact that our previous study found that silver nanoparticles caused only minor non-significant changes in liver and kidney function tests. 8,23. Furthermore, silver nanoparticles caused less pathogenic changes than silver ions, as demonstrated by Qin et al. 21 , kim et al. 22 and Adeyemi et al. 23. While Silver nitrates caused liver, kidney, and lung damage in both the dams and their offspring. Notably, zinc chloride treatment reduced silver nitrate cytotoxicity, as demonstrated by biochemical and histological changes in the liver and kidneys that were identical to the control group. In rats, zinc showed a protective effect against silver-induced damage. 14. Similarly, zinc increased the antioxidant state of nickel-treated rats’ livers 15. Zinc also protected the liver and kidneys from mercury-induced damage 16, 17.
Acknowledgment
We grateful thanks for Eman Abdulrahim for help during carrying out the experimental research in forensic medicine and toxicology department, faculty of veterinary medicine , Mansoura university.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Source
The
authors declare that there is no funding source.
References
- Gao X, Yourick JJ, Topping VD, Black T, Olejnik N, Keltner Z, et al. Toxicogenomic study in rat thymus of F1 generation offspring following maternal exposure to silver ion. Toxicology Reports. 2015;2:341-50.
CrossRef - Turner RD, Wingham JR, Paterson TE, Shepherd J, Majewski C. Use of silver-based additives for the development of antibacterial functionality in Laser Sintered polyamide 12 parts. Scientific Reports. 2020;10(1):1-11.
CrossRef - Boateng J, Catanzano O. Silver and silver nanoparticle‐based antimicrobial dressings. Therapeutic Dressings and Wound Healing Applications. 2020:157-84.
CrossRef - Marambio-Jones C, Hoek EM. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research. 2010;12(5):1531-51.
CrossRef - Smith I, Carson B. Trace metals in the environment. Vol. 2. Silver. Ann Arbor Science Publishers, Inc, P O Box 1425, Ann Arbor, Mich(Available in the U K from John Wiley & Sons Ltd, Baffins Lane, Chichester, London) 1977, 469 p. 1977.
- Ratte HT. Bioaccumulation and toxicity of silver compounds: a review. Environmental Toxicology and Chemistry: An International Journal. 1999;18(1):89-108.
CrossRef - Murthy G, Rhea U. Cadmium and silver content of market milk. Journal of Dairy Science. 1968;51(4):610-3.
CrossRef - Elalfy MM, Abouelmagd M, Abdelraheem EA, El-hadidy MG. Hepatorenal Effects of Silver Nanoparticles in In-Vivo Postnatal Model of Toxicity and in HepG2 Cell Line. Material Science Research India. 2020;17(1):54-61.
CrossRef - Elalfy M, El-hadidy M, Abouelmagd M. Update of usefulness and adverse effects of nanoparticles on animals and human health. J Vet Med Health. 2018;2:104.
- Fehaid A, Hamed MF, Abouelmagd MM, Taniguchi AJAJN. Time-dependent toxic effect and distribution of silver nano-particles compared to silver nitrate after intratracheal instillation in rats. 2016;4:12-9.
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14(3-4):331-41.
CrossRef - Ziemińska E, Strużyńska L. Zinc modulates nanosilver-induced toxicity in primary neuronal cultures. Neurotoxicity Research. 2016;29(2):325-43.
CrossRef - Minghetti M, Schirmer K. Interference of silver nanoparticles with essential metal homeostasis in a novel enterohepatic fish in vitro system. Environmental Science: Nano. 2019;6(6):1777-90.
CrossRef - Salazar-García S, Delgado-Buenrostro NL, Rodríguez-Escamilla JC, Davalos-Rivas G, Chirino YI, del Campo CGCM, et al. Zinc protects the rat brain from damage induced by 24 h exposure to silver nanoparticles. Journal of Nanoparticle Research. 2019;21(8):172.
CrossRef - Samir D, Kechrid Z, Djabar MR. Combined protective effect of zinc and vitamin C on nickel-induced oxidative liver injury in rats. Annals of Biological research. 2012;3(7):3410-8.
- Mesquita M, Pedroso TF, Oliveira CS, Oliveira VA, do Santos RF, Bizzi CA, et al. Effects of zinc against mercury toxicity in female rats 12 and 48 hours after HgCl2 exposure. EXCLI journal. 2016;15:256.
- Jamakala O, Rani UA. Amelioration effect of zinc and iron supplementation on selected oxidative stress enzymes in liver and kidney of cadmium-treated male albino rat. Toxicology International. 2015;22(1):1.
CrossRef - Olechnowicz J, Tinkov A, Skalny A, Suliburska JJTJoPS. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. 2018;68(1):19-31.
CrossRef - Alderman TS, Carpenter CB, McGirr R. Animal Research Biosafety. Applied Biosafety. 2018;23(3):130-42.
CrossRef - Bancroft John D, Alan S. Theory and Practice of histological techniques. Churchill Livingstone Elsevier. 2002;7.
- Qin G, Tang S, Li S, Lu H, Wang Y, Zhao P, et al. Toxicological evaluation of silver nanoparticles and silver nitrate in rats following 28 days of repeated oral exposure. Environmental toxicology. 2017;32(2):609-18.
CrossRef - Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhalation toxicology. 2008;20(6):575-83.
CrossRef - Adeyemi OS, Adewumi I, Faniyan TO. Silver nanoparticles influenced rat serum metabolites and tissue morphology. Journal of Basic and Clinical Physiology and Pharmacology. 2015;26(4):355-61.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Sara T. Elazab2, Mona G. Elhadidy3
, Sara T. Elazab2, Mona G. Elhadidy3 and Mahmoud M. Elalfy1*
and Mahmoud M. Elalfy1*


 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal





