Investigation on Solid Polymer Electrolyte (SPE) Membrane of Composition [(1-X) PEO: x AgCl] Prepared by Hot Press Technique
Mohan L.Verma1 and Arti Verma2
1Condensed Matter Physics Research Laboratory, Department of Applied Physics, Shri Shankaracharya College of Engineering and Technology - Junwani, Bhilai- 490 020, India.
2Department of Applied Physics, Rungta College of Engineering and Technology, Bhilai,Chhatisgarh, India.
DOI : http://dx.doi.org/10.13005/msri/090208
Article Publishing History
Article Received on : 08 Jun 2012
Article Accepted on : 14 Jul 2012
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Solid polymer Electrolyte (SPE ) is prepared by using PEO as host polymer and AgCl as salt in appropriate wt% ratio of the composition [(1-X) PEO: x AgCl ] where X = 10, 20, 30, 40 etc.by novel hot press technique. For ion transport property study conductivity (s) measurement is done for various composition x in (wt %) and the composition [70 PEO: 30 AgCl] is identified as optimum conducting composition (OCC). The variation in conductivity as a function of temperature is analysed and activation energy (Ea) is computed be least square fitting of the data of conductivity with respect to temperature. The conductivity enhancement of more than one order is obtained in SPE (OCC) as compare to pure PEO. The morphological and structural characterization of SPE membrane is done by using XRD/DSC, SEM, and FTIR studies.
KEYWORDS:
Activation energy; Hot press technique; Ionic conductivity; Polymer electrolyte
Copy the following to cite this article:
Verma M. L, Verma A. Investigation on Solid Polymer Electrolyte (SPE) Membrane of Composition [(1-X) PEO: x AgCl] Prepared by Hot Press Technique. Mat.Sci.Res.India;9(2)
|
Copy the following to cite this URL:
Verma M. L, Verma A. Investigation on Solid Polymer Electrolyte (SPE) Membrane of Composition [(1-X) PEO: x AgCl] Prepared by Hot Press Technique. Mat.Sci.Res.India;9(2). Available from: http://www.materialsciencejournal.org/?p=1122
|
Introduction
Composite electrolyte systems are the new class of fast ion conductors or super ionic solids. Super ionic are solids which contains high degree of ionic conductivity and low degree of electronic conductivity. Solid polymer electrolyte (SPE) membrane is emerging as candidates to be used as electrolyte membranes in high energy applications1 viz. solid state batteries, memory devices, chemical sensors, etc because of their safety performance, ease of handling, long life high processibility.2-3 To improve the properties of polymer electrolytes like mechanical stability, electrical conductivity, flexibility etc physical and chemical modifications are followed over the year have resulted in variety of polymer electrolytes. In chemical modification co-polymerization of main chain with co-monomer, cross-linking by chemical reaction or irradiation or thermal treatment and formation of interpenetrating network are the some of the methods used.4
In physical modification methods morphology and phase structure of polymer electrolytes can be changed by incorporating a suitable plasticizer or organic/inorganic filler which either introduces flexibility in polymeric chain or creates amorphous cell with defectation around dispersed grains.5 The addition of small organic/ inorganic or inert fillers decreases the crystallinity and enhances amorphousity of system.6 The conductivity of polymer electrolytes is affected by grain size distribution, particle concentration, surface area and phase structucture. Higher conductivity can be obtained by using nanosize fillers, which are much more prone to agglomeration than microsize fillers.7
Composite electrolytes are prepared adopting various procedures depending upon types of fillers and the form of polymeric material.8 Most of the researcher uses solution cast method for the preparation of polymer composite. A solvent free dry method known as hot press technique9 is recently being used for the preparation of polymeric membrane. This technique is quicker as well as cost effective.10
The present paper reports the casting of Ag+ ion conducting SPE membrane [(1-x) PEO: xAgCl] by novel hot press technique. The conductivity of SPE membrane for various compositions is measured. The transport properties are studied with the help of ionic conductivity (σ) at room temperature. The morphological characterization is done with the help of XRD, SEM and FTIR study. For the thermal characterization DSC is done.
Experimental
Material Preparations
For the casting of Ag+ ion conducting solid polymer electrolyte (SPE) membrane [(1-x) PEO: x AgCl] where x=10, 20, 30, 40 etc in (wt%) ratio solvent free / dry hot press technique is used. For the casting of membrane precursor grade chemicals PEO poly(ethylene oxide) (106 Mw, Aldrich, USA) and silver chloride AgCl (purity>99%, Reidel Lab Reagent) are used. Powders of constituent chemicals in appropriate (wt %) ratio is homogeneously mixed for 30 min in an agate pestle & mortar at room temperature. Finely grounded mixture of different compositions is heated separately nearly at temperature~ 70oC (close to melting point of PEO) with continuously mixing for 20 minutes. The soft slurry so obtained is then pressed between two cold SS- blocks (~ 1.25 tons/ cm2) which give rise to film of uniform thickness 0.035 cm.
Conductivity (Σ) Measurement
Conductivity (σ) measurements are carried out on samples of different composition by keeping them between two non blocking silver electrodes at fixed frequency (~1kHz) using LCR Bridge (ESCORT , ELC-131D, TAIWAN). Activation energy (Ea) was determined by least square fitting of conductivity data obtained at different temperature and for all the compositions of SPE membrane.
Material Characterization Studies
For the phase identification of SPE (OCC) XRD is used. For thermal analysis of the specimen DSC is used. For structural and morphological characterization SEM study is performed.
Results and Discussion
Measurement of Ionic Conductivity (s) as A Function of Salt Concentration and Temperature
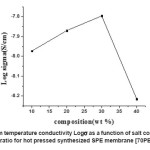
Figure1 depicts the salt concentration dependent room temperature conductivity (σ) of solid polymer electrolyte (SPE) membrane [(1-x) PEO: xAgCl] where x is take as 10, 20, 30, 40 in wt % ratio. From the figure it is clear that ionic conductivity increases gradually with the increase in salt concentration (x) in wt % ratio it attains maximum value for x=30(wt %)of salt concentration and then starts decreasing with further increase in salt concentration. The maximum conductivity (σ) obtained for x= 30 wt % of salt concentration is optimum conducting composition (OCC) of the SPE membrane. The SPE film beyond 50(wt %) ratio of salt concentration becomes less stable, brittle and sticky. The SPE membrane of composition [70PEO:30AgCl] with room temperature conductivity of (σ = 1.6×10-8 S/cm) with (σ) enhancement of approximately one order of magnitude can be seen as compare pure PEO (polymeric host).
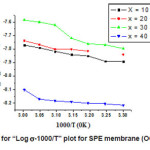
Fig. 2 depicts the temperature dependence of Ionic conductivity (logs-1000/T) of SPE membrane. The behavior is typical Arrhenius behavior described by
σ(T)=σ0 exp(-Ea/kT)
Where σ0 is pre exponential factor and Ea is activation energy
The conductivity of SPE (OCC) membrane increases linearly with temperature. From the figure it is clear that there is sudden jump at (~ 700C) of conductivity which corresponds to semicrystalline to amorphous phase transition of PEO. The linear portion below this temperature may be expressed by Arrhenius equation:
SPE Host Membrane
σ (T) =0.494×10-4 exp (-0.814/kT)
Where activation energy (Ea) = 0.814eV for the SPE (OCC) membrane is calculated by least square fitting of the data of conductivity
Morphological/Structural Characterization Phase Identification: X- Ray Diffraction (XRD) Study
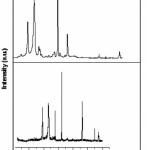
Figure3 depicts the XRD patterns for pure PEO and SPE (OCC) membrane of composition [70PEO:30AgCl]. As compare to diffractogram (a) of pure PEO, XRD patterns of SPE (OCC) exhibits appearance of some of the prominent peaks which are diminished and shifted toward higher 2è side reflecting characteristic features of semicrystalline/ amorphous polymers.11-12
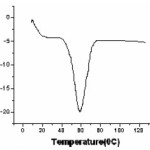
Thermal Analysis: Differential Scanning Calorimetric (DSC)
Figure4 shows DSC thermogram of synthesized OCC Solid Polymer electrolyte (SPE). In polymeric materials crystalline and amorphous phase coexists and thus are expected to give rise to occurrence of both glass transition endotherm at Tg (characteristic of amorphous phase) and melting endotherm at Tm (characteristic of crystalline phase).13 But in present work SPE (OCC) exhibits only melting endotherm of around ~60oC. The lowering of melting point of SPE (OCC) indicates partial complexation of salt AgCl with PEO. Occurrence of single peak indicates homogeneous distribution of these complexes
Scanning Electron Microscopy (SEM)
Figure5 depicts the SEM image of SPE (OCC) membrane; from the figure it is clear that the surface of SPE OCC is smooth. On the surface in some part we can see agglomerates of salts which shows the characteristics of the polymeric host.
Infrared Spectrum Analysis (FTIR) Study
Figure7 (a) & (b) depicts IR spectrum of pure PEO and SPE (OCC) [70PEO:30AgCl] respectively in absorption mode.The FTIR spectrum of synthesized SPE (OCC) confirms the formation of polymer- salt complex.
Conclusion
A new Ag+ ion conducting SPE membrane [70PEO:30AgCl] has been synthesized by novel hot press technique. The optimum conducting composition (OCC) [70PEO:30AgCl] is determined by compositional variation of conductivity. Theion conduction mechanism is explained on the basis of transport parameters viz ionic conductivity (s), ionic transference number (t ion ~ o.95) which indicates that SPE membrane is predominantly ion conducting.
The activation energy (Ea~0.817 ev) of SPE (OCC) is computed by least square linear fitting of data of conductivity with respect to temperature. The structural and morphological characterization of the membrane is done with the help of SEM and FTIR.
For the phase identification XRD is used and for the thermal analysis DSC is done, DSC thermogram indicates a sharp melting point of polymeric host PEO in SPE (OCC) membrane. This thin film of SPE(OCC) can be used to fabricate thin film batteries.
Acknowledgements
The Authors are thankful to Chhattisgarh council of science and technology, Raipur for providing financial assistance through the mini research project (Lett.N0.-356/CCOST/MRP/09-04- 08-09), to UGC-DAE consortium for scientific research Indore for providing lab facility and Dr. R.C. Agrawal, solid state Ionics lab, Raipur for providing valuable guidance.
References
- P.Bruce , Solid State Electrochemistry, Cambidge,Univ. Press, Cambridge (1995).
- J.A. MacCallum and C.A Vincent , Polymer Electrolyte Review- I Elsevier, London (1987).
- M.Stephan, European Polymer Journal, 21: 42 (2006).
- P.M.Blonsky, D.F Shrives, P.Austin and H.R Allcock,Solid State Ionics 18: 258 (1986)
CrossRef
- G.Zukowska,J.Williams,J.R Stevans,K.R Jeffrey, L.Lewera and P.J Kulesza,Solid State Ionics 167: 123-1130 (2004).
CrossRef
- F.Croce, B.Scrosati,G. Mariotto, Chem. Matter. 4: 1134 (1992).
CrossRef
- F.Croce, G.B appeticchi, L.Perci, B.Scrosati, Nature 394: 456 (1998).
CrossRef
- M. Marcinek, A.Bac, P.Lipka, A.Zalewska, G.Zukowska, R.Bokowska, W.Wiezorek, J. Phys. Chem. B 1004: 11088 (2000).
CrossRef
- F.Croce, R. Curini, A.Martinello, L. Persi, F. Ronci, B. Scrosati, R. Caminiti, J. Phys. Chem. 103: 10632 (1999).
CrossRef
- R.C. Agrawal, Angesh Chandra,J.Physics.D Appl. Phy. 40: 7024 (2007).
- B.d.cullity,Elements of X-ray diffraction, Addison Wesley Pub.Co.USA (1978).
- S.L. Agrawal and P.K Shukla, Ind. J.of Pure and Appl.Phys. 38: 53 (2000).
- A.Tager , Physicakl Chemistry of Polymers, MIR Publisher, Moscow (1978).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal