Experimental and Theoretical Studies on the Molecular Structure, FT-IR, NMR, HOMO, LUMO, MESP, and Reactivity Descriptors of (E)-1-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one
Introduction
Chalcones are open chain compounds that are considered as pathway to naturally occurring compounds flavonoids and isoflavonoids. Many chalcones and their hybrid derivatives are synthesized in last two decades.1-5 Both natural and synthetic versions of chalcones display enormous applications in pharmacological field.6-10 Structurally, chalcones are 1,3-diaryl-2-propen-1-ones and exist as cis and trans forms; latter being thermodynamically more stable is more predominant. Chalcones are considered as vital intermediates to synthesized wide variety of heterocyclic compounds with broad-spectrum of biological activities.11-16 The extraordinary pharmacological profile of chalcones and their hybrid derivatives include anticancer,17-20 anti-tubercular,21,22 antibacterial,23-26 anti-HIV,27-30 antimalarial,31-34 antioxidants,35-37 antiviral,38-40 antifungal,41-45 cardiovascular,46,47 antitumor,48-50 antiulcer,51 anticonvulsant,52, 53 anti-inflammatory,54-59 activities. Besides, chalcones also exhibit applications in the field of solar cells,60 photo-alignment layer of liquid crystal display,61 optoelectronics,62 corrosion and photo crosslinking,63 and nonlinear optical materials.64-67 All these noteworthy aspects about chalcones make them widely studied and synthesized compounds in the fields of science. Green chemistry has advanced in recent years and many methods have been modified in order increase the reaction efficiency and reduce waste material.68-75 Many methods have been accounted for the synthesis of chalcones; nut still, the most common method which is being used for the synthesis of chalcones is Claisen-Schmidt condensation.5
The field of DFT has attracted researchers due to its wide applications in structural chemistry. Many vital structural parameters could be anticipated with the help of DFT. The molecular properties like molecular structure, bond lengths, and bond angles along with spectroscopic properties like UV-Visible, FT-IR, Raman, and NMR have been largely explored by using DFT method using proper basis set.76-80 To investigate all these important aspects DFT has been employed to study the molecules like 2-arylidene indanone,81 (E)-1-(5-bromo-2-hydroxybenzylidene)semicarbazide,82 (E)-1-(4-bromobenzylidene)semicarbazide,83 3,5-Difluoroaniline,84 (E)-3-[4-(pentyloxy)phenyl]-1-phenylprop-2-en-1-one,85 N-phenylbenzenesulfonamide,86 3-ethynylthiophene,87 SnO2 nanopowder,88 2-Thienylboronic acid,89 3,5-dimethyl-4-methoxybenzoic acid,90 4-hydroxy-3-methoxycinnamaldehyde,91 3– alkyl–4–[3–methoxy–4–(4–methylbenzoxy)benzylidenamino]–4,5–dihydro–1H–1,2,4–triazol–5–ones,92 5-bromo-2-ethoxyphenylboronic acid,93 5-benzyl 2-thiohydantoin,94 Chlorfenson,95 sulfamethoxazole,96 terephthalic acid,97 1-phenyl-2-nitropropene,98 1-bromo-4-nitrobenzene,99 4-bromo-1-(ethoxycarbonyl)piperidine-4-carboxylic acid,100 2-Bromo-1H-Benzimidazol,101 dansyl chloride 102 etc. In view of all these discussed vital aspects of the modern times, herein I wish report combined experimental and theoretical studies on the molecular structure, FT-IR, NMR, HOMO, LUMO, MESP surface, and reactivity descriptors of (E)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one. In the present research, DFT investigation of the optimized molecular structure, bond length, bond angle, Mulliken atomic charges and harmonic vibrational frequencies have been investigated. The important parameters such as total energy, HOMO-LUMO energies, charge distribution, ionization potential, electron affinity, electronegativity, global softness, absolute hardness, global electrophilicity index, chemical potential, charge transfer have been studied using 6-311++G(d,p) basis set.
Materials and Methods
General Remarks
The chemicals (Make- Sigma Aldrich, SD Fine Pvt. Ltd., and Avra synthesis) were purchased from local distributor, Nashik with a high purity. The FT-IR spectrum of the DTMPP was recorded on Shimadzu spectrometer using a KBr disc technique. The NMR experiment was performed on sophisticated multinuclear FT-NMR Spectrometer (500 MHz) model Advance-II (Bruker). The compound was dissolved in chloroform-d. Chemical shifts were reported in ppm relative to tetramethylsilane (TMS). The reaction was followed by using thin-layer chromatography on Merck Aluminium TLC plate, silica gel coated with fluorescent indicator F254. All the glass apparatus were cleaned and dried in oven prior to use.
Experimental procedure for the synthesis of the DTMPP
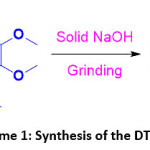
The DTMPP was synthesized using Claisen-Schmidt condensation reaction. In a typical synthesis method, 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)ethan-1-one (1, 10 mmol) and 3,4,5-trimethoxybenzaldehyde (1, 10 mmol) were mixed and added into mortar and pestle. Equimolar amount of solid NaOH was added. Then the alkaline mixture was grinded until formation of the product. After completion of the reaction (monitored by TLC), the reaction was quenched by pouring onto the crushed ice. It was then acidified by dilute HCl, filtered, dried and recrystallized to give pure crystals of the DTMPP. The reaction is presented in the Scheme 1.
Scheme 1: Synthesis of the DTMPP
Computational Details
For DFT calculations Gaussian 03(W) program package is used. All the calculations are performed at optimized by using DFT/B3LYP method using 6-311++G(d,p) basis set. Gauss View 4.1 molecular visualization program is used to visualize the optimized structure. The molecular structure is optimized at the same level. The structural parameters like bond length and bond angles, Mulliken atomic charges, molecular electrostatic surface potential, thermochemical data for the DTMPP were determined. The absorption wavelength (λ in nm), oscillator strength (ƒ), and transitions of DTMPP were computed at TD-B3LYP/6-311++G(d,p) level of theory for B3LYP/6-311G++(d,p) optimized geometry. All the DFT calculations were performed for the optimized molecular structure in the gas phase.
Results and Discussion
Spectral analysis of the DTMPP
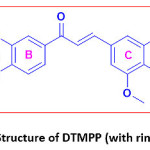
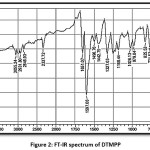
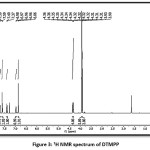
The DTMPP was characterized by spectral methods like FTIR, 1H NMR, and 13C NMR. The structure with ring labelling is depicted in Figure 1. The FT-IR spectrum is depicted in Figure 2, 1H NMR in Figure 3, and 13C NMR in Figure 4. In 1H NMR spectrum, the DTMPP has displayed all expected signal. The two protons situated at the C=C (alkene framework) are mutually coupled to each other by a coupling constant, J = 15.6 Hz which suggests the stereochemistry of an olefinic double bond is trans. All other signals in 1H NMR spectrum are ideally matching with structural arrangement of the DTMPP. In 13C NMR spectrum, the very important carbon signal is at 188.49 δ which is ascribed to ketonic carbonyl carbon. Other carbon signals are also correctly matching. The FT-IR spectral assignments were discussed in latter section.
Figure 1: Structure of DTMPP (with ring labeling)
(E)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one
FT-IR (cm-1, KBr) 3055.24, 2931.80, 2846.93, 2337.72, 1651.07, 1597.06, 1496.76, 1442.75, 1327.03, 1180.44, 1026.13, 979.84, 825.53, 771.53, 686.66, 555.50, 516.92; 1H NMR (500 MHz, CDCl3) δ 3.90 (s, 3H), 3.93 (s, 6H), 4.37 – 4.30 (m, 4H), 6.86 (s, 2H), 6.99 – 6.94 (m, 1H), ), 7.38 (d, J = 15.6 Hz, 1H), 7.60 (m, 2H), 7.71 (d, J = 15.6 Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 56.23, 61.04, 64.20, 64.73, 105.53, 117.36, 118.05, 121.02, 122.70, 130.55, 131.98, 140.23, 143.40, 144.32, 147.93, 153.47, 188.49.
Figure 2: FT-IR spectrum of DTMPP
Figure 3: 1H NMR spectrum of DTMPP
Figure 4: 13C NMR spectrum of DTMPP
DFT Study
Molecular structure, bond length, bond angle study
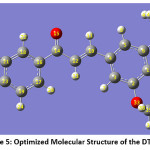
The optimized molecular structure of the DTMPP has been presented in the Figure 5. Optimized bond lengths and bond angles of DTMPP at B3LYP/6-311++G(d,p) are presented in Table 1 and Table 2 respectively. The DTMPP possess aromatic C=C bond lengths from 1.39 Å to 1.40 Å. The alkene (C10=C12) bond is 1.3447 Å long and the carbonyl (C14-O15) bond is 1.2249 Å in length. The C1-C6 bond is the longest (1.4089 Å) C=C bond in the DTMPP. The oxygen atom O33 is having short contact interaction with the hydrogen atom H44 with a distance of 3.3258 Å. The dipole moment of the DTMPP is 1.27 Debye with C1 point group symmetry and -1225.77 a.u. E(B3LYP) energy. The bond angles C23-O32-C28, C21-O31-C25, C25-C28-O32, and C28-C25-O31 are 114.3374, 113.7255, 110.1429, and 110.0488° respectively. Other bond length and bond angle data is also in good agreement.
Figure 5: Optimized Molecular Structure of the DTMPP
Table 1: Optimized bond lengths of DTMPP at B3LYP/6-311++G(d,p)
|
Bond lengths (Å)
|
|
C1-C2
|
1.403
|
C12-C14
|
1.4843
|
C25-C28
|
1.5166
|
|
C1-C6
|
1.4089
|
C14-O15
|
1.2249
|
C25-O31
|
1.428
|
|
C1-C9
|
1.3684
|
C14-C16
|
1.4986
|
C28-H29
|
1.096
|
|
C2-C3
|
1.3905
|
C16-C17
|
1.4034
|
C28-H30
|
1.0904
|
|
C2-O33
|
1.3712
|
C16-C18
|
1.4002
|
C28-O32
|
1.4321
|
|
C3-C4
|
1.3999
|
C17-C19
|
1.3882
|
O33-C35
|
1.4344
|
|
C3-H7
|
1.0822
|
C17-H20
|
1.0813
|
O33-H44
|
3.3258
|
|
C4-C5
|
1.4066
|
C18-C21
|
1.3848
|
O34-C39
|
1.4224
|
|
C4-C10
|
1.4599
|
C18-H22
|
1.0824
|
C35-H36
|
1.0896
|
|
C5-C6
|
1.3927
|
C19-C23
|
1.393
|
C35-H37
|
1.0911
|
|
C5-H8
|
1.0815
|
C19-H24
|
1.0833
|
C35-H38
|
1.0957
|
|
C6-C34
|
1.3626
|
C21-C23
|
1.4073
|
C39-H40
|
1.0886
|
|
O9-C43
|
1.435
|
C21-C31
|
1.3731
|
C39-H41
|
1.0954
|
|
C10-H11
|
1.0874
|
C23-C32
|
1.3667
|
C39-H42
|
1.0951
|
|
C10-C12
|
1.3447
|
C25-H26
|
1.0905
|
C43-H44
|
1.0944
|
|
C12-H13
|
1.0817
|
C25-H27
|
1.0968
|
C43-H45
|
1.0921
|
|
–
|
–
|
–
|
–
|
C43-H44
|
1.0896
|
Table 2: Optimized bond angles of DTMPP at B3LYP/6-311++G(d,p)
|
Bond angles (°)
|
|
C2-C1-C6
|
119.203
|
C15-C14-C16
|
119.9414
|
C25-C28-O32
|
110.1429
|
|
C2-C1-O9
|
119.654
|
C14-C16-C17
|
124.1855
|
C29-C28-H30
|
109.2448
|
|
C6-C1-O9
|
121.1151
|
C14-C16-C18
|
117.105
|
C29-C28-O32
|
109.4186
|
|
C1-C2-C3
|
120.5619
|
C17-C16-C18
|
118.7092
|
H30-C28-O32
|
106.2509
|
|
C1-C2-O33
|
120.8758
|
C16-C17-C19
|
120.5274
|
C21-O31-C25
|
113.7255
|
|
C3-C2-O33
|
118.4956
|
C16-C17-H20
|
121.0765
|
C23-O32-C28
|
114.3374
|
|
C2-C3-C4
|
120.6212
|
C19-C17-H20
|
118.3819
|
C2-O33-C35
|
115.9901
|
|
C2-C3-H7
|
117.6039
|
C16-C18-C21
|
121.0782
|
C6-O34-C39
|
118.3398
|
|
C4-C3-H7
|
121.7635
|
C16-C18-H22
|
119.3046
|
O33-C35-H36
|
105.9214
|
|
C3-C4-C5
|
118.8277
|
C21-C18-H22
|
119.617
|
O33-C35-H37
|
111.2063
|
|
C3-C4-C10
|
123.211
|
C17-C19-C23
|
120.381
|
O33-C35-H38
|
110.4968
|
|
C5-C4-C10
|
117.9597
|
C17-C19-H24
|
121.508
|
H36-C35-H37
|
109.8833
|
|
C4-C5-C6
|
120.9288
|
C23-C19-H24
|
118.1089
|
H36-C35-H38
|
109.2885
|
|
C4-C5-H8
|
118.6506
|
C18-C21-C23
|
119.7267
|
H37-C35-H38
|
109.9568
|
|
C6-C5-H8
|
120.4205
|
C18-C21-O31
|
118.7515
|
O34-C39-H40
|
105.7252
|
|
C1-C6-C5
|
119.851
|
C23-C21-O31
|
121.5209
|
O34-C39-H41
|
111.5441
|
|
C1-C6-O34
|
115.4102
|
C19-C23-C21
|
119.5634
|
O34-C39-H42
|
111.3529
|
|
C5-C6-O34
|
124.7374
|
C19-C23-O32
|
118.4071
|
H40-C39-H41
|
109.3594
|
|
C1-O9-C43
|
115.3767
|
C21-C23-O32
|
122.0292
|
H40-C39-H42
|
109.3165
|
|
C4-C10-H11
|
116.1274
|
H26-C25-H27
|
109.1376
|
H41-C39-H42
|
109.4515
|
|
C4-C10-C12
|
128.0884
|
H26-C25-C28
|
111.3025
|
O9-C43-H44
|
110.546
|
|
C11-C10-C12
|
115.7842
|
H26-C25-O31
|
106.4255
|
O9-C43-H45
|
110.8797
|
|
C10-C12-H13
|
120.8568
|
H27-C25-C28
|
110.1061
|
O9-C43-H46
|
106.0459
|
|
C10-C12-C14
|
119.9991
|
H27-C25-O31
|
109.746
|
H44-C43-H45
|
110.1828
|
|
H13-C12-C14
|
119.1389
|
C28-C25-O31
|
110.0488
|
H44-C43-H46
|
109.5231
|
|
C12-C14-O15
|
120.986
|
C25-C28-H29
|
110.1292
|
H45-C43-H46
|
109.573
|
|
C12-C14-C16
|
119.0706
|
C25-C28-H30
|
111.5645
|
–
|
–
|
Mulliken atomic charges and MESP analysis
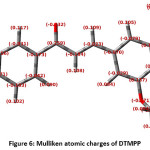
The Mulliken nuclear charges depend on the electron density. The charge conveyance on the molecule has a fundamental job in the field of quantum mechanical calculations for the molecular systems. The Mulliken atomic charges of the DTMPP are determined by DFT/B3LYP method with a 6-311++G(d,p) basis set are given in Table 3 and the pictorial presentation in Figure 6. Mulliken nuclear charges uncover that all the hydrogen atoms have a net positive charge but H40 atom has a more positive charge (0.131720) than other hydrogen atoms and in this way highly acidic. The C14 atom has the highest net positive charge (0.258693) and the C16 is the most electronegative carbon (-0.172547). Molecular electrostatic potential surface (MESP) is the three dimensional portrayal of the charge distributions in the molecules.
Figure 6: Mulliken atomic charges of DTMPP
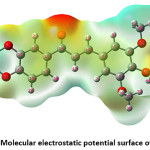
The MESP diagram plotted by employing 6-311++G(d,p) basis set and depicted in Figure 7.81 Over the span of recent years, the molecular electrostatic potential has risen as a persuading tool for exploring molecular interactions. In current science, it has been adequately associated with a wide assortment of biological and chemical platforms. The vital aspects such as nucleophilic and electrophilic reactivity sites, solvent interactions, hydrogen-bonding phenomenon, and non-classical interactions could be anticipated by studying MESP plots. MESP fundamentally used to point out the reactive sites of molecules that enable us to foresee how one molecule can interface with others. The red and yellow colours in the MESP plot indicate the region of high electron density and therefore linked with electrophilic reactivity. On the contrary, the blue colours reveal low electron density and thus susceptible to nucleophilic attacks. The green colours are areas of zero potential. The MESP plot of the DTMPP suggest that the electrophilic attacks are feasible at both aromatic rings; However, ring C is more prone for the attack of electrophiles. The positive potential is around hydrogen atoms.
Table 3: Mulliken atomic charges of DTMPP
|
Atom
|
Charge
|
Atom
|
Charge
|
|
1 C
|
0.128758
|
24 H
|
0.101714
|
|
2 C
|
0.137002
|
25 C
|
-0.039861
|
|
3 C
|
-0.028609
|
26 H
|
0.122036
|
|
4 C
|
-0.070414
|
27 H
|
0.125682
|
|
5 C
|
-0.078696
|
28 C
|
-0.033079
|
|
6 C
|
0.186623
|
29 H
|
0.128327
|
|
7 H
|
0.084342
|
30 H
|
0.121675
|
|
8 H
|
0.105487
|
31 O
|
-0.347037
|
|
9 O
|
-0.373934
|
32 O
|
-0.347018
|
|
10 C
|
-0.022806
|
33 O
|
-0.370983
|
|
11 H
|
0.108857
|
34 O
|
-0.352687
|
|
12 C
|
-0.183709
|
35 C
|
-0.112558
|
|
13 H
|
0.108190
|
36 H
|
0.112704
|
|
14 C
|
0.258693
|
37 H
|
0.126112
|
|
15 O
|
-0.332374
|
38 H
|
0.093758
|
|
16 C
|
-0.172547
|
39 C
|
-0.134310
|
|
17 C
|
-0.042463
|
40 H
|
0.131720
|
|
18 C
|
-0.030810
|
41 H
|
0.115056
|
|
19 C
|
-0.102820
|
42 H
|
0.117146
|
|
20 H
|
0.090052
|
43 C
|
-0.106943
|
|
21 C
|
0.156429
|
44 H
|
0.103928
|
|
22 H
|
0.117097
|
45 H
|
0.116446
|
|
23 C
|
0.171490
|
46 H
|
0.114333
|
Figure 7: Molecular electrostatic potential surface of DTMPP
HOMO, LUMO, reactivity descriptors, and absorption energies
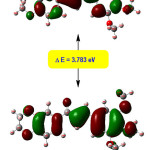
The pictorial representation of the frontier molecular orbitals (FMOs) has depicted in Figure 8. The FMOs, HOMO and LUMO are crucial to evaluate the reactivity ad the stability of the molecules. More importantly HOMO is connected to the electron donating capacity and the LUMO is connected with electron-accepting ability. The smaller HOMO-LUMO band gap suggests more stability. Thus, the HOMO-LUMO energy gap is the most important indicator of the kinetic stability of molecules. The HOMO and LUMO energies are -6.023 and -2.280 eV individually. The reduction in the HOMO-LUMO energy gap prompts an expansion in polarizability, flexibility, and electron transport in a molecule. The HOMO and LUMO energies are extremely essential as they are linked to ionization enthalpy and electron affinity individually. The HOMO in the DTMPP is principally located at ring B and C. The LUMO is basically situated at the enone part of the unsaturated system. The energy gap in the DTMPP is 3.783 eV which reveals inevitable charge transfer phenomena taking place within the molecule.
Figure 8: HOMO-LUMO pictures of DTMPP
With the help of Koopmans’s theorem, various reactivity descriptors are calculated. The important parameters such as total energy, HOMO-LUMO energies, charge distribution, electron affinity, ionization potential, global softness, absolute hardness, electronegativity, global electrophilicity index, chemical potential, and charge transfer are evaluated. The ionization potential (I) and the electron affinity (A) values are 6.023 and 2.280 eV respectively. The electronegativity value in electron volt is 4.15 eV. The absolute hardness (ɳ) is 1.89 eV and the global softness (σ) is 0.53 eV-1. These values indicate that the molecule DTMPP is softer in nature. The global electrophilicity index (ω) is 4.55 eV which tells that the DTMPP is a good electrophile. The chemical potential (Pi) value is -4.15 eV in the DTMPP. The maximum charge transfer (ΔNmax) in the DTMPP is 2.19 eV.
The absorption wavelength (λ in nm), oscillator strength (ƒ), and transitions of DTMPP were computed at TD-B3LYP/6-311++ G(d,p) level of theory for B3LYP/6-311G ++(d,p) optimized geometry in gas phase presented in the Table 4. The UV-Visible spectrum was computed for six excited states. The first excited state absorption wavelength is 375.83 nm with excitation energy of 3.2990 eV and oscillator strength (ƒ), 0.0035. With increase in the number of excited state, there is decrease in the absorption wavelength and increase in the excitation energy.
Table 4: Absorption energies (λ in nm), Oscillator strength (ƒ), and transitions of DTMPP computed at TD-B3LYP/6-311G++(d,p) level of theory for B3LYP/6-311G ++(d, p) optimized geometry in gas phase
|
Sr. No.
|
Absorption Wavelength (nm)
|
Excitation energy (eV)
|
Excited state
|
Oscillator strength (f)
|
|
1
|
375.83
|
3.2990
|
1
|
0.0035
|
|
2
|
363.45
|
3.4113
|
2
|
0.6667
|
|
3
|
340.53
|
3.6409
|
3
|
0.0441
|
|
4
|
325.29
|
3.8115
|
4
|
0.1847
|
|
5
|
311.35
|
3.9821
|
5
|
0.0338
|
|
6
|
265.39
|
4.6718
|
6
|
0.0812
|
Vibrational assignments and thermochemical study
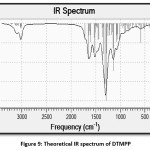
The vibrational assignments for the DTMPP were made by comparing experimental IR spectrum with the theoretical IR spectrum. The DFT based IR absorption values are slightly higher, therefore a scaling factor of 0.96 is used.81 A Comparison between selected theoretical and experimental vibrational assignments has been made and presented in Table 5. The DTMPP contains 46 atoms; therefore, it shows 132 fundamental modes of vibrations.Results indicate that there is ideal matching between experimental and theoretical vibrational peaks. The theoretical IR spectrum is given in Figure 9. The DTMPP has experimental carbonyl vibrational stretching frequency at 1651.07 cm-1. The C=C of enone framework has stretching absorption peak at 1597.06 cm-1. The peak at 3055.24 cm-1 is due to stretching of C12-H bond, asymmetric stretching of C17-H-C19-H and C39-H2 bonds. The IR peak at 1442.75 cm-1 corresponds to scissoring vibration of C39-H2 bond. The vibrational peak at 1327.03 cm-1 is due to wagging vibrations of C25-H2 and C28-H2 bonds. The IR value 1180.44 cm-1 is due to in plane bending vibrations of C17-H, C18-H, and C19-H bonds. The out of plane bending vibration of C5-H bond is observed at 825.53 cm-1. The absorption peak 555.50 cm-1 is ascribed to deformation vibrations of ring A, B, and ring C. Other vibrational assignments were also correctly made using theoretical IR spectrum.
Figure 9: Theoretical IR spectrum of DTMPP
Table 5: Comparison between selected experimental and theoretical vibrational assignments calculated at 6-311++G(d,p) level
|
Mode
|
Computed frequencies (cm-1)
|
IR Intensity (km) mol-1
|
Observed frequencies (cm-1)
|
Assignments
|
|
127
|
3059.26
|
5.01
|
3055.24
|
v C12-H
+ ν asym C17-H-C19-H
|
|
118
|
2944.62
|
31.79
|
2931.80
|
ν asym C39-H2
|
|
113
|
2887.07
|
51.43
|
2846.93
|
ν sym C39-H2
|
|
112
|
1648.18
|
156.75
|
1651.07
|
ν C=O
|
|
111
|
1583.68
|
338.02
|
1597.06
|
ν C10=C12
|
|
106
|
1471.56
|
124.61
|
1496.76
|
ν C=C (ring B)
|
|
102
|
1444.08
|
77.89
|
1442.75
|
ν scis C39-H2
|
|
90
|
1337.20
|
1.59
|
1327.03
|
ω C25-H2 + ω C28-H2
|
|
85
|
1259.33
|
462.73
|
–
|
t C25-H2
|
|
83
|
1238.51
|
28.43
|
–
|
ρ C17-H-C19-H
|
|
78
|
1176.00
|
81.98
|
1180.44
|
β C17-H + β C18-H + β C19-H
|
|
65
|
1030.44
|
113.60
|
1026.13
|
ν C25-O31
|
|
62
|
983.50
|
100.86
|
979.84
|
def ring C
|
|
51
|
815.11
|
24.10
|
825.53
|
γ C5-H
|
|
49
|
790.41
|
31.37
|
771.53
|
ω C17-H-C19-H
|
|
44
|
694.67
|
5.74
|
686.66
|
def ring B
|
|
38
|
559.07
|
65.24
|
555.50
|
def ring A + def ring B + def ring C
|
v– stretching; sym- symmetric; asym- asymmetric; def- deformation; scis- scissoring β- In-plane bending; γ- out of plane bending; ρ- rocking; t- twisting, -wagging
The thermochemistry data obtained for the DTMPP from the DFT method at B3LYP/6-311++G(d,p) level is presented in Table 6. In the present study, Etotal, Heat capacity at constant volume, total entropy S, zero point vibrational energy and rotational constants have been evaluated from harmonic vibrational frequencies. The total thermal energy is 244.365 kcal mol-1. The total entropy is 178.630 cal mol-1K-1; out of which translational freedom is
43.504, rotational is 36.445, and the vibrational is 98.681 cal mol-1K–1. The zero point vibrational energy is 228.84140 Kcal mol-1. The information uncovered in this could be helpful for the further evaluation of the other thermodynamic properties.
Table 6: Thermochemical information of DTMPP
|
Parameter
|
Value
|
|
E total (kcal mol-1)
Translational
Rotational
Vibrational
|
244.365
0.889
0.889
242.587
|
|
Heat Capacity at constant volume,
Cv (cal mol-1K-1)
Translational
Rotational
Vibrational
|
91.704
2.981
2.981
85.742
|
|
Total entropy S
(cal mol-1K-1)
Translational
Rotational
Vibrational
|
178.630
43.504
36.445
98.681
|
|
Zero point Vibrational Energy Ev0 (Kcal mol-1)
|
228.84140
|
|
Rotational constants (GHZ)
|
0.53604 0.06036 0.05497
|
Conclusion
In outline, the current research deals with the synthesis, characterization and DFT study of (E)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (DTMPP). For the computational investigation, DFT method at B3LYP/6-311++G(d,p) basis set has been used.
- Structural properties like molecular structure, bond lengths, and bond angles of the DTMPP have been investigated. The DTMPP has aromatic C=C bond lengths from 1.39 Å to 1.40 Å. The alkene (C10=C12) bond is 1.3447 Å long and the carbonyl (C14-O15) bond is 1.2249 Å in length. The C1-C6 bond is the longest (1.4089 Å) C=C bond in the DTMPP. The oxygen atom O33 is having short contact interaction with the hydrogen atom H44 with a distance of 3.3258 Å.
- Mulliken atomic charges uncover that all hydrogen atoms possess net positive charge but H40 atom has a more positive charge (0.131720) than other hydrogen atoms and thusly exceptionally acidic. The C14 atom has the most noteworthy net positive charge (0.258693) and the C16 is the most electronegative carbon (- 0.172547).
- The MESP plot of the DTMPP proposes that the electrophilic attacks are feasible at both aromatic rings; However, ring C is more prone to the attack of electrophiles. The positive potential is around hydrogen atoms.
- The HOMO in the DTMPP is essentially situated at ring B and C. The LUMO is fundamentally arranged at the enone part of the unsaturated framework. The energy gap in the DTMPP is 3.783 eV which uncovers inescapable charge transfer phenomena occuring within the molecule.
- The absorption wavelength, oscillator strength, and transitions of DTMPP were computed at TD-B3LYP/6-311++G(d,p) level of theory for B3LYP/6-311G ++(d,p) basis set. The UV-Visible spectrum was computed for six excited states. The first excited state absorption wavelength is 375.83 nm with excitation energy of 3.2990 eV and oscillator strength (ƒ), 0.0035. With increase in the number of excited state, there is decrease in the absorption wavelength and increase in the excitation energy.
- The vibrational assignments for the DTMPP were made by comparing the experimental IR spectrum with the theoretical IR spectrum. The DTMPP contains 46 atoms; along these lines, it shows 132 fundamental modes of vibrations. The vibrational outcome shows that there is a perfect matching between experimental and theoretical vibrational peaks.
- Etotal, heat capacity at constant volume, total entropy, zero point vibrational energy and rotational constants have been evaluated from harmonic vibrational frequencies.
Acknowledgments
Authors acknowledge Central instrumentation facility, Savitribai Phule Pune University, Pune for NMR and CIC, KTHM College, Nashik for FT-IR spectral analysis. Authors also would like to thank principal of Arts, Science and Commerce College, Manmad, for permission and providing necessary research facilities. Authors are very grateful to Prof. Arun B. Sawant his generous help in the Gaussian guidance. Dr. Aapoorva Prashant Hiray, Coordinator, MG Vidyamandir Institute, is gratefully acknowledged for Gaussian package.
Funding
We have not received any kind of fund for the research work and this work is self-funded.
Conflict of Interest
Authors declared that he do not have any conflict of interest regarding this research article.
References
- Li, J.T., Yang, W.Z., Wang, S.X., Li, S.H. and Li, T.S., 2002. Improved synthesis of chalcones under ultrasound irradiation. Ultrasonics Sonochemistry, 9(5), pp.237-239.
- Eddarir, S., Cotelle, N., Bakkour, Y. and Rolando, C., 2003. An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron letters, 44(28), pp.5359-5363.
- Kumar, A., Sharma, S., Tripathi, V.D. and Srivastava, S., 2010. Synthesis of chalcones and flavanones using Julia–Kocienski olefination. Tetrahedron, 66(48), pp.9445-9449.
- Jioui, I., Dânoun, K., Solhy, A., Jouiad, M., Zahouily, M., Essaid, B., Len, C. and Fihri, A., 2016. Modified fluorapatite as highly efficient catalyst for the synthesis of chalcones via Claisen–Schmidt condensation reaction. Journal of Industrial and Engineering Chemistry, 39, pp.218-225.
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sagane, A.A., 2020. Ultrasound promoted stereoselective synthesis of 2,3-dihydrobenzofuran appended chalcones at ambient temperature. South African Journal of Chemistry, 73, pp.35-43.
- Rammohan, A., Reddy, J.S., Sravya, G., Rao, C.N. and Zyryanov, G.V., 2020. Chalcone synthesis, properties and medicinal applications: a review. Environmental Chemistry Letters, pp.1-26.
- Yang, J.L., Ma, Y.H., Li, Y.H., Zhang, Y.P., Tian, H.C., Huang, Y.C., Li, Y., Chen, W. and Yang, L.J., 2019. Design, Synthesis, and Anticancer Activity of Novel Trimethoxyphenyl-Derived Chalcone-Benzimidazolium Salts. ACS omega, 4(23), pp.20381-20393.
- Burmaoglu, S., Yilmaz, A.O., Polat, M.F., Kaya, R., Gulcin, İ. and Algul, O., 2019. Synthesis of novel tris-chalcones and determination of their inhibition profiles against some metabolic enzymes. Archives of physiology and biochemistry, pp.1-9.
- Aljamali, N., Hamzah Daylee, S. and Jaber Kadhium, A., 2020. Review on Chemical-Biological Fields of Chalcone Compounds. Forefront Journal of Engineering &Technology, 2(1), pp.33-44.
- Rozmer, Z. and Perjési, P., 2016. Naturally occurring chalcones and their biological activities. Phytochemistry reviews, 15(1), pp.87-120.
- Yadav, L.D.S., Patel, R., Rai, V.K. and Srivastava, V.P., 2007. An efficient conjugate hydrothiocyanation of chalcones with a task-specific ionic liquid. Tetrahedron letters, 48(44), pp.7793-7795.
- MURAoKA, O., SAwADA, T., MoRIMoTo, E. and TANABE, G., 1993. Chalcones as synthetic intermediates. A facile route to (±)-magnosalicin, an antiallergy neolignan. Chemical and pharmaceutical bulletin, 41(4), pp.772-774.
- Kalirajan, R., Sivakumar, S.U., Jubie, S., Gowramma, B. and Suresh, B., 2009. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res, 1(1), pp.27-34.
- Elarfi, M.J. and Al-Difar, H.A., 2012. Synthesis of some heterocyclic compounds derived from chalcones. Scientific Reviews and Chemical Communications, 2(2), pp.103-107.
- MT Albuquerque, H., MM Santos, C., AS Cavaleiro, J. and MS Silva, A., 2014. Chalcones as Versatile Synthons for the Synthesis of 5-and 6-membered Nitrogen Heterocycles. Current Organic Chemistry, 18(21), pp.2750-2775.
- Kidwai, M. and Misra, P., 1999. Ring closure reactions of chalcones using microwave technology. Synthetic communications, 29(18), pp.3237-3250.
- Yadav, P., Lal, K., Kumar, A., Guru, S.K., Jaglan, S. and Bhushan, S., 2017. Green synthesis and anticancer potential of chalcone linked-1, 2, 3-triazoles. European journal of medicinal chemistry, 126, pp.944-953.
- Zhao, L., Mao, L., Hong, G., Yang, X. and Liu, T., 2015. Design, synthesis and anticancer activity of matrine–1H-1, 2, 3-triazole–chalcone conjugates. Bioorganic & medicinal chemistry letters, 25(12), pp.2540-2544.
- Rai, U.S., Isloor, A.M., Shetty, P., Pai, K.S.R. and Fun, H.K., 2015. Synthesis and in vitro biological evaluation of new pyrazole chalcones and heterocyclic diamides as potential anticancer agents. Arabian Journal of Chemistry, 8(3), pp.317-321.
- Madhavi, S., Sreenivasulu, R., Yazala, J.P. and Raju, R.R., 2017. Synthesis of chalcone incorporated quinazoline derivatives as anticancer agents. Saudi Pharmaceutical Journal, 25(2), pp.275-279.
- Mujahid, M., Yogeeswari, P., Sriram, D., Basavanag, U.M.V., Díaz-Cervantes, E., Córdoba-Bahena, L., Robles, J., Gonnade, R.G., Karthikeyan, M., Vyas, R. and Muthukrishnan, M., 2015. Spirochromone-chalcone conjugates as antitubercular agents: synthesis, bio evaluation and molecular modeling studies. RSC Advances, 5(129), pp.106448-106460.
- Singh, A., Viljoen, A., Kremer, L. and Kumar, V., 2018. Synthesis and antimycobacterial evaluation of piperazyl‐alkyl‐ether linked 7‐chloroquinoline‐chalcone/ferrocenyl chalcone conjugates. ChemistrySelect, 3(29), pp.8511-8513.
- Chu, W.C., Bai, P.Y., Yang, Z.Q., Cui, D.Y., Hua, Y.G., Yang, Y., Yang, Q.Q., Zhang, E. and Qin, S., 2018. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. European journal of medicinal chemistry, 143, pp.905-921.
- Vazquez-Rodriguez, S., López, R.L., Matos, M.J., Armesto-Quintas, G., Serra, S., Uriarte, E., Santana, L., Borges, F., Crego, A.M. and Santos, Y., 2015. Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorganic & medicinal chemistry, 23(21), pp.7045-7052.
- Alam, M.S., Rahman, S.M. and Lee, D.U., 2015. Synthesis, biological evaluation, quantitative-SAR and docking studies of novel chalcone derivatives as antibacterial and antioxidant agents. Chemical Papers, 69(8), pp.1118-1129.
- Tang, X., Su, S., Chen, M., He, J., Xia, R., Guo, T., Chen, Y., Zhang, C., Wang, J. and Xue, W., 2019. Novel chalcone derivatives containing a 1, 2, 4-triazine moiety: design, synthesis, antibacterial and antiviral activities. RSC advances, 9(11), pp.6011-6020.
- Hameed, A., Abdullah, M.I., Ahmed, E., Sharif, A., Irfan, A. and Masood, S., 2016. Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential non-nucleoside reverse transcriptase inhibitors (NNRT). Bioorganic chemistry, 65, pp.175-182.
- Wu, J.H., Wang, X.H., Yi, Y.H. and Lee, K.H., 2003. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorganic & medicinal chemistry letters, 13(10), pp.1813-1815.
- Rizvi, S.U.F., Siddiqui, H.L., Johns, M., Detorio, M. and Schinazi, R.F., 2012. Anti-HIV-1 and cytotoxicity studies of piperidyl-thienyl chalcones and their 2-pyrazoline derivatives. Medicinal Chemistry Research, 21(11), pp.3741-3749.
- Singh, P., Anand, A. and Kumar, V., 2014. Recent developments in biological activities of chalcones: a mini review. European journal of medicinal chemistry, 85, pp.758-777.
- Wu, X., Wilairat, P. and Go, M.L., 2002. Antimalarial activity of ferrocenyl chalcones. Bioorganic & medicinal chemistry letters, 12(17), pp.2299-2302.
- Li, R., Kenyon, G.L., Cohen, F.E., Chen, X., Gong, B., Dominguez, J.N., Davidson, E., Kurzban, G., Miller, R.E., Nuzum, E.O. and Rosenthal, P.J., 1995. In vitro antimalarial activity of chalcones and their derivatives. Journal of medicinal chemistry, 38(26), pp.5031-5037.
- Domínguez, J.N., León, C., Rodrigues, J., de Domínguez, N.G., Gut, J. and Rosenthal, P.J., 2005. Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Il Farmaco, 60(4), pp.307-311.
- Hans, R.H., Guantai, E.M., Lategan, C., Smith, P.J., Wan, B., Franzblau, S.G., Gut, J., Rosenthal, P.J. and Chibale, K., 2010. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorganic & medicinal chemistry letters, 20(3), pp.942-944.
- Lahsasni, S.A., Al Korbi, F.H. and Aljaber, N.A.A., 2014. Synthesis, characterization and evaluation of antioxidant activities of some novel chalcones analogues. Chemistry Central Journal, 8(1), pp.1-10.
- Aly, M.R.E.S., Fodah, H.H.A.E.R. and Saleh, S.Y., 2014. Antiobesity, antioxidant and cytotoxicity activities of newly synthesized chalcone derivatives and their metal complexes. European journal of medicinal chemistry, 76, pp.517-530.
- Wang, G., Xue, Y., An, L., Zheng, Y., Dou, Y., Zhang, L. and Liu, Y., 2015. Theoretical study on the structural and antioxidant properties of some recently synthesised 2, 4, 5-trimethoxy chalcones. Food chemistry, 171, pp.89-97.
- Wan, Z., Hu, D., Li, P., Xie, D. and Gan, X., 2015. Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules, 20(7), pp.11861-11874.
- Zhou, D., Xie, D., He, F., Song, B. and Hu, D., 2018. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorganic & medicinal chemistry letters, 28(11), pp.2091-2097.
- Wang, Y.J., Zhou, D.G., He, F.C., Chen, J.X., Chen, Y.Z., Gan, X.H., Hu, D.Y. and Song, B.A., 2018. Synthesis and antiviral bioactivity of novel chalcone derivatives containing purine moiety. Chinese Chemical Letters, 29(1), pp.127-130.
- Konduru, N.K., Dey, S., Sajid, M., Owais, M. and Ahmed, N., 2013. Synthesis and antibacterial and antifungal evaluation of some chalcone based sulfones and bisulfones. European journal of medicinal chemistry, 59, pp.23-30.
- Parikh, K. and Joshi, D., 2013. Antibacterial and antifungal screening of newly synthesized benzimidazole-clubbed chalcone derivatives. Medicinal Chemistry Research, 22(8), pp.3688-3697.
- Kulkarni, R.R., Tupe, S.G., Gample, S.P., Chandgude, M.G., Sarkar, D., Deshpande, M.V. and Joshi, S.P., 2014. Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Natural product research, 28(4), pp.245-250.
- Zheng, Y., Wang, X., Gao, S., Ma, M., Ren, G., Liu, H. and Chen, X., 2015. Synthesis and antifungal activity of chalcone derivatives. Natural product research, 29(19), pp.1804-1810.
- de Sá, N.P., Cisalpino, P.S., Tavares, L.D.C., Espíndola, L., Pizzolatti, M.G., Santos, P.C., de Paula, T.P., Rosa, C.A., de Souza, D.D.G., Santos, D.A. and Johann, S., 2015. Antifungal activity of 6-quinolinyl N-oxide chalcones against Paracoccidioides. Journal of Antimicrobial Chemotherapy, 70(3), pp.841-845.
- Mahapatra, D.K. and Bharti, S.K., 2016. Therapeutic potential of chalcones as cardiovascular agents. Life sciences, 148, pp.154-172.
- Opletalova, V., Jahodar, L., Jun, D. and Opletal, L., 2003. Chalcones (1, 3-diarylpropen-1-ones) and their analogs as potential therapeutic agents in cardiovascular system diseases. Ceska a Slovenska farmacie: casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti, 52(1), pp.12-19.
- Kumar, S.K., Hager, E., Pettit, C., Gurulingappa, H., Davidson, N.E. and Khan, S.R., 2003. Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. Journal of medicinal chemistry, 46(14), pp.2813-2815.
- Kumar, D., Kumar, N.M., Akamatsu, K., Kusaka, E., Harada, H. and Ito, T., 2010. Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorganic & medicinal chemistry letters, 20(13), pp.3916-3919.
- Xia, Y., Yang, Z.Y., Xia, P., Bastow, K.F., Nakanishi, Y. and Lee, K.H., 2000. Antitumor agents. Part 202: novel 2′-amino chalcones: design, synthesis and biological evaluation. Bioorganic & medicinal chemistry letters, 10(8), pp.699-701.
- Sashidhara, K.V., Avula, S.R., Mishra, V., Palnati, G.R., Singh, L.R., Singh, N., Chhonker, Y.S., Swami, P. and Bhatta, R.S., 2015. Identification of quinoline-chalcone hybrids as potential antiulcer agents. European journal of medicinal chemistry, 89, pp.638-653.
- Sharma, C.S., Shekhawat, K.S., Chauhan, C.S. and Kumar, N., 2013. Synthesis and anticonvulsant activity of some chalcone derivatives. Journal of Chemical and Pharmaceutical Research, 5(10), pp.450-454.
- Singh, N., Ahmad, S. and Alam, M.S., 2012. Biological potentials of chalcones: a review. International Journal of Pharmaceutical and Biological Archives, 3(6), pp.1298-1303.
- Mahapatra, D.K., Bharti, S.K. and Asati, V., 2017. Chalcone derivatives: Anti-inflammatory potential and molecular targets perspectives. Current topics in medicinal chemistry, 17(28), pp.3146-3169.
- Bhale, P.S., Chavan, H.V., Dongare, S.B., Shringare, S.N., Mule, Y.B., Nagane, S.S. and Bandgar, B.P., 2017. Synthesis of extended conjugated indolyl chalcones as potent anti-breast cancer, anti-inflammatory and antioxidant agents. Bioorganic & medicinal chemistry letters, 27(7), pp.1502-1507.
- Reddy, M.V.B., Hung, H.Y., Kuo, P.C., Huang, G.J., Chan, Y.Y., Huang, S.C., Wu, S.J., Morris-Natschke, S.L., Lee, K.H. and Wu, T.S., 2017. Synthesis and biological evaluation of chalcone, dihydrochalcone, and 1, 3-diarylpropane analogs as anti-inflammatory agents. Bioorganic & medicinal chemistry letters, 27(7), pp.1547-1550.
- Wen, R., Lv, H.N., Jiang, Y. and Tu, P.F., 2018. Anti-inflammatory flavone and chalcone derivatives from the roots of Pongamia pinnata (L.) Pierre. Phytochemistry, 149, pp.56-63.
- Wang, L., Yang, X., Zhang, Y., Chen, R., Cui, Y. and Wang, Q., 2019. Anti-inflammatory Chalcone–Isoflavone Dimers and Chalcone Dimers from Caragana jubata. Journal of Natural Products, 82(10), pp.2761-2767.
- Nowakowska, Z., 2007. A review of anti-infective and anti-inflammatory chalcones. European journal of medicinal chemistry, 42(2), pp.125-137.
- Makhlouf, M.M., Radwan, A.S. and Ghazal, B., 2018. Experimental and DFT insights into molecular structure and optical properties of new chalcones as promising photosensitizers towards solar cell applications. Applied Surface Science, 452, pp.337-351.
- Song, D.M., Jung, K.H., Moon, J.H. and Shin, D.M., 2003. Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display. Optical Materials, 21(1-3), pp.667-671.
- Chaudhry, A.R., Irfan, A., Muhammad, S., Al-Sehemi, A.G., Ahmed, R. and Jingping, Z., 2017. Computational study of structural, optoelectronic and nonlinear optical properties of dynamic solid-state chalcone derivatives. Journal of Molecular Graphics and Modelling, 75, pp.355-364.
- Ramaganthan, B., Gopiraman, M., Olasunkanmi, L.O., Kabanda, M.M., Yesudass, S., Bahadur, I., Adekunle, A.S., Obot, I.B. and Ebenso, E.E., 2015. Synthesized photo-cross-linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies. RSC Advances, 5(94), pp.76675-76688.
- Indira, J., Karat, P.P. and Sarojini, B.K., 2002. Growth, characterization and nonlinear optical property of chalcone derivative. Journal of crystal growth, 242(1-2), pp.209-214.
- Poornesh, P., Shettigar, S., Umesh, G., Manjunatha, K.B., Kamath, K.P., Sarojini, B.K. and Narayana, B., 2009. Nonlinear optical studies on 1, 3-disubstituent chalcones doped polymer films. Optical materials, 31(6), pp.854-859.
- Shettigar, S., Chandrasekharan, K., Umesh, G., Sarojini, B.K. and Narayana, B., 2006. Studies on nonlinear optical parameters of bis-chalcone derivatives doped polymer. Polymer, 47(10), pp.3565-3567.
- Ravindra, H.J., Kiran, A.J., Chandrasekharan, K., Shashikala, H.D. and Dharmaprakash, S.M., 2007. Third order nonlinear optical properties and optical limiting in donor/acceptor substituted 4′-methoxy chalcone derivatives. Applied Physics B, 88(1), pp.105-110.
- Adole, V.A., Pawar, T.B., Koli, P.B. and Jagdale, B.S., 2019. Exploration of catalytic performance of nano-La 2 O 3 as an efficient catalyst for dihydropyrimidinone/thione synthesis and gas sensing. Journal of Nanostructure in Chemistry, 9(1), pp.61-76.
- Ritter, M., Martins, R.M., Rosa, S.A., Malavolta, J.L., Lund, R.G., Flores, A.F. and Pereira, C.M., 2015. Green synthesis of chalcones and microbiological evaluation. Journal of the Brazilian Chemical Society, 26(6), pp.1201-1210.
- Rateb, N.M. and Zohdi, H.F., 2009. Atom-efficient, solvent-free, green synthesis of chalcones by grinding. Synthetic Communications®, 39(15), pp.2789-2794.
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2019. Aqua‐mediated rapid and benign synthesis of 1, 2, 6, 7‐tetrahydro‐8H‐indeno [5,4‐b] furan‐8‐one‐appended novel 2‐arylidene indanones of pharmacological interest at ambient temperature. Journal of the Chinese Chemical Society.67:306-315.
- Vieira, Lucas CC, Márcio Weber Paixão, and Arlene G. Corrêa. “Green synthesis of novel chalcone and coumarin derivatives via Suzuki coupling reaction.” Tetrahedron Letters 53, no. 22 (2012): 2715-2718.
- Romanelli, G., Pasquale, G., Sathicq, Á., Thomas, H., Autino, J. and Vázquez, P., 2011. Synthesis of chalcones catalyzed by aminopropylated silica sol–gel under solvent-free conditions. Journal of Molecular Catalysis A: Chemical, 340(1-2), pp.24-32.
- Rani, M.S., Rohini, C., Keerthi, B.S. and Mamata, C., 2019. Green Synthesis of Novel Chalcone Derivatives, Characterization and its Antibacterial Activity. Research Journal of Science and Technology, 11(3), pp.183-185.
- Adole, V.A., More, R.A., Jagdale, B.S., Pawar, T.B. and Chobe, S.S., 2020. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2‐(2‐Hydrazineyl) thiazole Derivatives. ChemistrySelect, 5(9), pp.2778-2786.
- Adole, V.A., Waghchaure, R.H., Jagdale, B.S. and Pawar, T.B., 2020. Investigation of Structural and Spectroscopic Parameters of Ethyl 4-(4-isopropylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: a DFT Study. Chemistry & Biology Interface, 10(1), 22-20.
- Selvaraj, S., Rajkumar, P., Kesavan, M., Thirunavukkarasu, K., Gunasekaran, S., Devi, N.S. and Kumaresan, S., 2020. Spectroscopic and structural investigations on modafinil by FT-IR, FT-Raman, NMR, UV–Vis and DFT methods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 224, p.117449.
- Pallavi, L., Tonannavar, J. and Tonannavar, J., 2020. DFT zwitterion model for vibrational and electronic structure of unnatural 3-amino-3-(4-fluorophenyl) propionic acid, aided by IR and Raman spectroscopy. Journal of Molecular Structure, p.128085.
- Ramesh, P., Caroline, M.L., Muthu, S., Narayana, B., Raja, M. and Aayisha, S., 2020. Spectroscopic and DFT studies, structural determination, chemical properties and molecular docking of 1-(3-bromo-2-thienyl)-3-[4-(dimethylamino)-phenyl] prop-2-en-1-one. Journal of Molecular Structure, 1200, p.127123.
- Iramain, M.A., Ledesma, A.E., Imbarack, E., Bongiorno, P.L. and Brandán, S.A., 2020. Spectroscopic studies on the potassium 1-fluorobenzoyltrifluoroborate salt by using the FT-IR, Raman and UV–Visible spectra and DFT calculations. Journal of Molecular Structure, 1204, p.127534.
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sawant, A.B., Experimental and theoretical exploration on single crystal, structural, and quantum chemical parameters of (E)‐7‐(arylidene)‐1,2,6,7‐tetrahydro‐8H‐indeno [5, 4‐b] furan‐8‐one derivatives: A comparative study. Journal of the Chinese Chemical Society. https://doi.org/10.1002/jccs.202000006.
- Raja, M., Muhamed, R.R., Muthu, S. and Suresh, M., 2017. Synthesis, spectroscopic (FT-IR, FT-Raman, NMR, UV–Visible), NLO, NBO, HOMO-LUMO, Fukui function and molecular docking study of (E)-1-(5-bromo-2-hydroxybenzylidene) semicarbazide. Journal of Molecular Structure, 1141, pp.284-298.
- Raja, M., Muhamed, R.R., Muthu, S. and Suresh, M., 2017. Synthesis, spectroscopic (FT-IR, FT-Raman, NMR, UV–Visible), first order hyperpolarizability, NBO and molecular docking study of (E)-1-(4-bromobenzylidene) semicarbazide. Journal of Molecular Structure, 1128, pp.481-492.
- Pathak, S.K., Srivastava, R., Sachan, A.K., Prasad, O., Sinha, L., Asiri, A.M. and Karabacak, M., 2015. Experimental (FT-IR, FT-Raman, UV and NMR) and quantum chemical studies on molecular structure, spectroscopic analysis, NLO, NBO and reactivity descriptors of 3, 5-Difluoroaniline. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 135, pp.283-295.
- Abbas, A., Gökce, H., Bahçeli, S. and Naseer, M.M., 2014. Spectroscopic (FT-IR, Raman, NMR and UV–vis.) and quantum chemical investigations of (E)-3-[4-(pentyloxy) phenyl]-1-phenylprop-2-en-1-one. Journal of Molecular Structure, 1075, pp.352-364.
- Govindarasu, K., Kavitha, E. and Sundaraganesan, N., 2014. Synthesis, structural, spectral (FTIR, FT-Raman, UV, NMR), NBO and first order hyperpolarizability analysis of N-phenylbenzenesulfonamide by density functional theory. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 133, pp.417-431.
- Karabacak, M., Bilgili, S., Mavis, T., Eskici, M. and Atac, A., 2013. Molecular structure, spectroscopic characterization (FT-IR, FT-Raman, UV and NMR), HOMO and LUMO analysis of 3-ethynylthiophene with DFT quantum chemical calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 115, pp.709-718.
- Ayeshamariam, A., Ramalingam, S., Bououdina, M. and Jayachandran, M., 2014. Preparation and characterizations of SnO2 nanopowder and spectroscopic (FT-IR, FT-Raman, UV–Visible and NMR) analysis using HF and DFT calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, pp.1135-1143.
- Sachan, A.K., Pathak, S.K., Sinha, L., Prasad, O., Karabacak, M. and Asiri, A.M., 2014. A combined experimental and theoretical investigation of 2-Thienylboronic acid: Conformational search, molecular structure, NBO, NLO and FT-IR, FT-Raman, NMR and UV spectral analysis. Journal of Molecular Structure, 1076, pp.639-650.
- Karabacak, M., Sinha, L., Prasad, O., Asiri, A.M., Cinar, M. and Shukla, V.K., 2014. FT-IR, FT-Raman, NMR, UV and quantum chemical studies on monomeric and dimeric conformations of 3, 5-dimethyl-4-methoxybenzoic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 123, pp.352-362.
- Thirunavukkarasu, K., Rajkumar, P., Selvaraj, S., Suganya, R., Kesavan, M., Gunasekaran, S. and Kumaresan, S., 2018. Vibrational (FT-IR and FT-Raman), electronic (UV–Vis), NMR (1H and 13C) spectra and molecular docking analyses of anticancer molecule 4-hydroxy-3-methoxycinnamaldehyde. Journal of Molecular Structure, 1173, pp.307-320.
- Gokce, H., Bahceli, S., Akyildirim, O., Yuksek, H. and Kol, O.G., 2013. The Syntheses, Molecular Structures, Spectroscopic Properties (IR, Micro–Raman, NMR and UV–vis) and DFT Calculations of Antioxidant 3–alkyl–4–[3–methoxy–4–(4–methylbenzoxy) benzylidenamino]–4, 5–dihydro–1H–1, 2, 4–triazol–5–one Molecules. Letters in Organic Chemistry, 10(6), pp.395-441.
- Sas, E.B., Kose, E., Kurt, M. and Karabacak, M., 2015. FT-IR, FT-Raman, NMR and UV–Vis spectra and DFT calculations of 5-bromo-2-ethoxyphenylboronic acid (monomer and dimer structures). Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, pp.1315-1333.
- Deval, V., Kumar, A., Gupta, V., Sharma, A., Gupta, A., Tandon, P. and Kunimoto, K.K., 2014. Molecular structure (monomeric and dimeric) and hydrogen bonds in 5-benzyl 2-thiohydantoin studied by FT-IR and FT-Raman spectroscopy and DFT calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 132, pp.15-26.
- Ramalingam, S., Periandy, S., Sugunakala, S., Prabhu, T. and Bououdina, M., 2013. Insilico molecular modeling, docking and spectroscopic [FT-IR/FT-Raman/UV/NMR] analysis of Chlorfenson using computational calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 115, pp.118-135.
- Chamundeeswari, S.V., Samuel, E.J.J. and Sundaraganesan, N., 2014. Molecular structure, vibrational spectra, NMR and UV spectral analysis of sulfamethoxazole. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, pp.1-10.
- Karthikeyan, N., Prince, J.J., Ramalingam, S. and Periandy, S., 2015. Electronic [UV–visible] and vibrational [FT-IR, FT-Raman] investigation and NMR–mass spectroscopic analysis of terephthalic acid using quantum Gaussian calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 139, pp.229-242.
- Xavier, S. and Periandy, S., 2015. Spectroscopic (FT-IR, FT-Raman, UV and NMR) investigation on 1-phenyl-2-nitropropene by quantum computational calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 149, pp.216-230.
- Shakila, G., Saleem, H. and Sundaraganesan, N., 2017. FT-IR, FT-Raman, NMR and UV Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic structure calculations of 1-bromo-4-nitrobenzene. World Scientific News, 61(2), pp.150-185.
- Vitnik, V.D. and Vitnik, Ž.J., 2015. The spectroscopic (FT-IR, FT-Raman, l3C, 1H NMR and UV) and NBO analyses of 4-bromo-1-(ethoxycarbonyl) piperidine-4-carboxylic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 138, pp.1-12.
- Sas, E.B., Kurt, M., Karabacak, M., Poiyamozhi, A. and Sundaraganesan, N., 2015. FT-IR, FT-Raman, dispersive Raman, NMR spectroscopic studies and NBO analysis of 2-Bromo-1H-Benzimidazol by density functional method. Journal of Molecular Structure, 1081, pp.506-518.
- Karabacak, M., Cinar, M., Kurt, M., Poiyamozhi, A. and Sundaraganesan, N., 2014. The spectroscopic (FT-IR, FT-Raman, UV and NMR) first order hyperpolarizability and HOMO–LUMO analysis of dansyl chloride. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 117, pp.234-244.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Vishnu Ashok Adole2*
, Vishnu Ashok Adole2* , Bapu Sonu Jagdale1,2
, Bapu Sonu Jagdale1,2 and Thansing Bhavsing Pawar1
and Thansing Bhavsing Pawar1
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal