Dhirendra Agarwal1, Neeraj Kumar2 and A. K. Bansal3
1Suresh Gyan ViharUniversity, Jaipur –302017 (Rajasthan), Jaipur –302017 (Rajasthan), Bharatpur-321001 (Rajasthan), India
2Mechanical Engineering Suresh Gyan Vihar university. Jaipur,
3S.G.V. Govt. Polytechnic College, Jaipur –302017 (Rajasthan), Jaipur –302017 (Rajasthan), Bharatpur-321001 (Rajasthan), India
Corresponding author Email: dhirendra_a@rediffmail.com
DOI : http://dx.doi.org/10.13005/msri/140215
Article Publishing History
Article Received on : 07 Dec 2017
Article Accepted on : 21 Dec 2017
Article Published : 25 Dec 2017
Plagiarism Check: Yes
Article Metrics
ABSTRACT:
Cast irons are basically binary alloys of iron and carbon having carbon exceeding its maximum solid solubility in austenite but less than the carbon content of iron carbide. However, like steels, cast irons have varying quantities of silicon, manganese, phosphorus and sulphur. Silicon plays an important role in controlling the properties of cast irons and for this reason, the term cast iron is usually applied to a series of iron, carbon and silicon alloys. Special purpose cast irons include white and alloy cast irons which are mainly used for applications demanding enhanced abrasion, corrosion or heat resistance. In present study, corrosion resistant cast irons are of our interest.
A detailed review of literature disclosed that corrosion resistant alloy cast irons currently in use is a compound of high percentage of silicon, nickel and chromium. Hence is called as chromium-molybdenum irons. It is widely used for oxidizing purpose. However, it shows low mechanical strength and shock resistance. Hence it is less useful for applications related with high mechanical strength and shocks. Ni-resist irons are useful in various types of ambient. It has low strength and become unsuitable at temperature higher than 780°C. High Ni- austenitic gray irons also suffer from corrosion due to graphite. High chromium irons is useful in all types of mold depending on chromium content. It demonstrates good resistance against attrition, abrasion and oxidation. The jostle resistivity of high chromium irons can be improved by reducing carbon content.
Low cost substitutes for these cast irons may be aimed at substituting the scarce elements namely nickel and/or molybdenum partly or fully. Nickel can be replaced partially by manganese and copper. Manganese also controls austenite like nickel completely. Hence it can be used to refine pearlite and promote the formation of any desired matrix (bainite or martensite or austenite) in the as-cast condition. It has a mild carbide-forming tendency. The carbide morphology of manganese bearing chromium irons was found to be similar to that observed in high chromium irons because both manganese and chromium have a similar carbide-forming tendency. Copper cannot fully stabilize austenite and is effective only in the presence of another austenite stabilizer.
The possibility of evolving low cost corrosion resistant white irons is based on the fact that the difference of electrochemical potential between austenite and graphite is higher than that between austenite and carbide and it is a reason of reduction in graphitic corrosion which is a typical problem in high alloyed corrosion resistance gray irons. The mixture of materials is preferred due to the fact that the elements are locally available and effective in production of corrosion resistant white irons. It is assumed that iron-manganese-chromium-copper white irons are capable to resist corrosion, wear and corrosive-wear.
The present study essentially comprised a detailed investigation of certain newly designed Fe-Mn-Cr-Mo white irons, containing 10% Mn-6% Cr-3.0% C,0.3%Mo, The investigation was aimed at developing cheaper corrosion resistant white cast irons having corrosion resistance similar to expensive highly alloyed Ni-Resist irons. The study comprised assessing the heat treatment response of the experimental alloy with a view to establish interrelation between structure and properties. Hardness measurements, optical and scanning metallography, quantitative metallography, electron probe micro analysis and differential thermal analysis were carried out to correlate structure, properties and corrosion rate. However corrosion rates in 6% NaCl solution were found comparable to that of Ni-Resist irons.
KEYWORDS:
ASTM; EPMA; LEITZ; Ni
Copy the following to cite this article:
Agarwal D, Kumar N, Bansal A. K. Development of Low Cost Corrosion Resistant Fe-Cr-Mn-Mo White Cast Irons. Mat.Sci.Res.India;14(2)
|
Copy the following to cite this URL:
Agarwal D, Kumar N, Bansal A. K. Development of Low Cost Corrosion Resistant Fe-Cr-Mn-Mo White Cast Irons. Mat.Sci.Res.India;14(2). Available from: http://www.materialsciencejournal.org/?p=6604
|
Introduction
These irons are hard, brittle and difficult to machine and therefore did not attract attention of metallurgists and materials engineers, as an engineering material for many years. Factors such as closer composition control of the melt, efficient use of alloying element(s), heat treatment as a tool in improving properties, availability of data related to service conditions, improved testing facilities raised the interest of researcher in this material.
The alloy white cast irons used for corrosion resistant applications are mostly high silicon irons, high chromium irons with or without molybdenum, and high nickel Ni-Resist irons. Although Ni-Resist irons, an expensive material due the presence of costly nickel in large amounts, are being extensively used in marine conditions, these exhibit low mechanical strength and suffer from graphitic corrosion and pitting- a severe form of corrosion. These also have limited application at high temperatures.
thats why Mo is used to enhance the hardnees and to reduce cost.
These shortcomings led to the development of corrosion resistant white cast irons. It is well-known fact that the single phase microstructure is most useful in resisting corrosion, though such materials possess low strength.
The effectiveness of a two phase microstructure in resisting corrosion depends on the morphology of the second phase, its volume fraction, nature of the matrix-second phase interface, and the difference in the electrochemical potentials of the two constituents. For example, an austenite – carbide couple is better than the ferrite – graphite couple in terms of resisting corrosion. For optimum corrosion resistance, second phase particles should be nearly spherical/ polygonal in shape.1 The volume fraction of the second phase should neither be too large nor too small. It is for the reasons that the larger volume fraction will enhance the galvanic action due to the increased interfacial area while the lesser can led to pitting corrosion. Second phase particles should preferably coherent as the coherent interface is stressed to minimum extent.
Multiphase microstructures are useful only when the presence of the third phase directly or indirectly helps in reducing the corrosion rate. Alloying elements adds to corrosion resistance by forming a passive film, changing the matrix phase, developing favourable morphology or by changing the electrochemical behaviour of the phases present.
White cast irons have no free carbon (graphite) and therefore are not susceptible to graphitic corrosion. These have better mechanical strength levels. A major advantage foreseen is that the corrosion resistance is expected to be better since austenite and carbide are less apart in the electrochemical series.2
Efforts have been made to develop corrosion resistant white cast iron based on the use of relatively low-cost and indigenously available alloying elements such as manganese, chromium and copper. The Fe- Mn- Cr-Mo alloy system was selected for the reasons .
(a) that the manganese enhances hardenability significantly at a low cost, stabilizes austenite as well as carbides. Additionally, it enhances castability- a property much needed for cast materials.
(b) that the chromium stabilizes carbides, helps in attaining a uniform structure with a minimum of segregation and is capable of generating martensite or austenite even if present singly in large proportions. Like manganese, it also raises the hardenability.3
Experimental Procedure
Composition of the alloy is given in Table 1. Heat treatments comprised of air cooling from 850,900, 950, and 1000°C after holding for periods ranging from 2 to 10 hours. Hardness testing was carried out on a Vickers hardness tester employing a 30 Kg load. Optical metallography was employed to study the effect of the heat treatment on microstructure. Specimens were etched with 2% natal. Quantitative metallography was carried out with the help of a LEITZ image analyzer and electron probe micro analyzer (EPMA). Corrosion testing was carried out as per the relevant ASTM specifications (G-1-72). Specimens, paper polished up to 4/0 to attain mirror finish, were cleaned firstly with double distilled water and finally with acetone, dried, weighed and suspended for 168 hours in a beaker containing 6% NaCl solution under stagnant conditions at room temperature. After removal from the solution, specimens were again cleaned, dried and weighed. From the weight loss, the corrosion rates in milligram per square decimeter per day (mdd) were calculated by using the formula 1:
Corrosion rate (in mdd) = K.W / (A.T.D)
Where
K = 2.4 x 106
W = weight loss in grams, to nearest 1 milligram.
A = surface area in square centimeter to nearest 0.01
square centimeter.
T = duration in hours to nearest 0.01 hour.
D = density of material in gram per cubic centimeter.
68th WFC – World Foundry Congress
The muffle furnace is used to prepare the material. the SEM machine at 3000x is used to capture the microstructure
|
C
|
S
|
P
|
Si
|
Mn
|
Cr
|
Mo
|
|
3.11
|
0.043
|
0.28
|
1.79
|
9.86
|
5.91
|
0.3
|
Table 2: Effect of heat treatment on hardness Alloy (As cast hardness= 565 HV30)
|
Temperature, deg. C
|
Hardness at soaking period in hours
|
|
2
|
4
|
6
|
8
|
10
|
|
800
|
536
|
532
|
532
|
528
|
525
|
|
850
|
528
|
523
|
515
|
497
|
491
|
|
900
|
521
|
518
|
505
|
498
|
488
|
|
950
|
498
|
488
|
480
|
472
|
469
|
|
1000
|
469
|
464
|
451
|
446
|
446
|
Table 3: Volume fraction of massive carbides Alloy (As cast = 42.7%)
|
Temperature, deg. C
|
Soaking period in hours
|
|
2
|
4
|
6
|
8
|
10
|
|
800
|
39.0
|
37.6
|
38.1
|
35.9
|
35.3
|
|
850
|
38.1
|
38.0
|
33.3
|
34.2
|
32.1
|
|
900
|
35.7
|
35.6
|
34.3
|
33.6
|
30.9
|
|
950
|
34.2
|
34.2
|
30.2
|
24.0
|
21.1
|
|
1000
|
28.0
|
28.9
|
26.2
|
21.4
|
16.8
|
Table 4: Effect of heat treatment on corrosion rate Alloy (As cast CR 23.588 mdd at 168 hrs. and 21.767 mdd at 720 hrs.)
|
Temper-
ature,
deg. C
|
2 hrs. SP
|
6 hrs. SP
|
10 hrs. SP
|
|
168 hrs.
|
720 hrs.
|
168 hrs.
|
720 hrs.
|
168 hrs.
|
720 hrs.
|
|
800
|
23.503
|
20.900
|
23.114
|
20.923
|
23.212
|
20.075
|
|
850
|
21.789
|
19.293
|
21.594
|
19.106
|
20.168
|
18.047
|
|
900
|
20.768
|
18.407
|
20.342
|
17.903
|
20.101
|
17.803
|
|
950
|
18.534
|
17.013
|
17.611
|
15.202
|
16.918
|
14.812
|
|
1000
|
16.052
|
14.334
|
16.019
|
13.792
|
15.823
|
13.372
|
Results
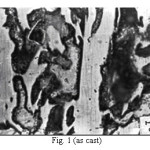
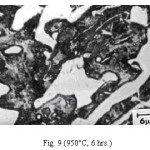
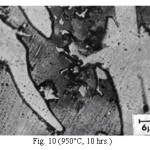
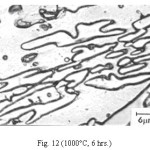
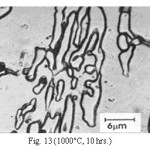
Representative microstructures of the alloy are shown in Figs. 1-13. The data on hardness, volume fraction of massive carbides and corrosion rate have been shown in Table 2, Table 3 and Table 4 respectively. The microstructure of the alloy in the as-cast condition (Fig. 1) essentially comprised of martensite + austenite + massive carbides. Volume fraction of massive carbides (platy carbides) was relatively large. The relatively high hardness of the as-cast alloy is consistent with its microstructure. Microstructures generated on heat treating from lower temperatures (specifically up to 950°C) were different from the as-cast one as these were comprised of additional dispersed phase having needle like and nearly spherical shaped particles. This dispersed phase was absent in the microstructures obtained on heat treating from higher temperatures. These microstructures (higher temperatureones) mainly comprised of austenite + massive carbides.
A critical analysis of hardness data contained in Tables and a perusal of the various Figures revealed that: Hardness was only marginally affected by the soaking period on air cooling from 800˚C for the alloy. Hardness decreased marginally up to 6 hours soaking period on air cooling from 850˚C beyond which, it decreased at relatively faster rate but not significantly. Hardness was only marginally affected by the soaking period on air cooling from 900˚C for the alloy. Hardness decreasing marginally with the soaking period on heat treating from 950˚C for the alloy.2 The Alloy showed that hardness is, for all practical purposes, is independent of soaking period at 1000˚C. Maximum and minimum resistance against softening with increasing soaking period at 1000˚C was shown by alloy. At 2 hours soaking period, Hardness minimum for alloy with increasing heat treatment temperature. At 4 hours soaking period, the same trend as observed for 2 hours was followed. At 6 hours soaking period, the alloy resist well against softening up to around 950˚C beyond which hardness decreased significantly. At 8 hours soaking period, Hardness decreased minimum for alloy with increasing heat treatment temperature. For the alloy, marked decrease was observed only on raising temperature above 900˚C.At 10 hours soaking period, the hardness trend was found to be similar as that for 8 hours soaking period. The hardness , in general , is decreasing with the soaking temperature in the order
Figure 1: (as cast)
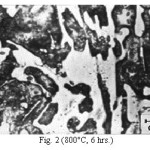
Figure 2: (800°C, 6 hrs.)
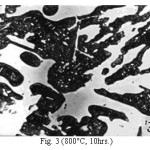
Figure 3: (800°C, 10hrs.)
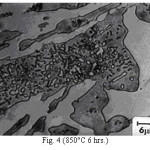
Figure 4: (850°C 6 hrs.)
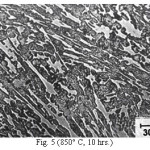
Figure 5: (850° C, 10 hrs.)
VHN1050 < VHN1000 < VHN950 < VHN900 < VHN850 < VHN800
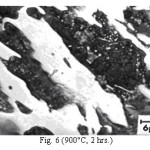
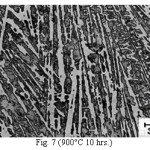
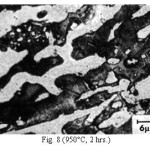
The microstructure (Figure 1) of the alloy in the as-cast condition is comprised of martensite, austenite and carbide. Massive carbides were present in large proportion. The carbide phase was found to have different morphologies. Precipitation of needle like phase, having mostly obtuse plate like appearance, was observed on heat treating from 800˚C for 2 hours. This needle like phase was existed at all soaking periods. On raising the soaking periods, presence of a phase in the form of unevenly dispersed spherical particles (Figures 2 & 3) was seen. This phase will be referred to as DSP in future. The microstructural features observed at 6 and 10 hours (Fig. 2 & 3) showed that the massive carbides rendered discontinuous. For 850˚C, 2 hours heat treatment, the microstructural features were similar to that observed at 800˚C except that much coarsening of both needle like phase and of DSP resulted. Similar features were seen on raising the soaking periods from 6 to 10 hours (Figures 4 & 5). Some of the salient features observed for 850˚C heat treatment temperature were: matrix mainly comprised of austenite, degree of coarsening increased with soaking period, coalescing of dispersed particles took place, disintegration of massive carbides occurred and needle ends got somewhat rounded. At 900˚C, 2 hours, marked decrease in the volume fraction of the needles like phase and disintegration of massive carbides were observed (Figure 6). On raising soaking periods, there was a general coarsening of DSP and continuous decrease in the volume fraction of massive carbides. At 10 hours soaking period, only a few needles were seen, massive carbides got disintegrated much, massive carbides acquired somewhat rounded morphology specifically at the ends and linking of massive carbides started (Figure 7). The microstructure of 950˚C, 2 hours heat treated alloy (Figure 8) resembles with that of 900˚C, 10 hours heat treated alloy. No significant change was observed in the basic features of microstructures obtained at higher soaking periods (6 & 10 hours). The feature of main interest was related to the morphology of massive carbides which were rendered spherical/ nearly spherical with round edges and ends (Figures 9 & 10). No needles were seen on heat treating from 1000˚C (Figures 11 & 12). At 2 hours soaking period (Figure 11), the disintegrating and rounding off tendency of massive carbides (as observed at 950˚C) continued. At higher soaking periods, the tendency of massive carbides towards disintegration and rounding off continued (Figures 12 & 13). Additionally, massive carbides aligned at higher soaking periods (Figures 12 & 13). Completely austenitic matrix was present.1
A perusal of the Table revealed that: Volume fraction of the massive carbides (VMC) in the as-cast condition was maximum for the alloy. For the alloy, volume fraction of the massive carbides decreased with an increase in the heat treatment temperature / time. At 800˚C, the alloy showed only marginal decrease in the volume fraction of massive carbides. At 850˚C, the alloy showed only marginal decrease in the volume fraction of massive carbides. At 900˚C, the behaviour of the alloy is similar to that observed at 850˚C. At 950˚C, the alloy, that was showing only marginal decrease up to 900˚C, showed a sharp decrease in the volume fraction of massive carbides. Reduction in the volume fraction of massive carbides was pronounced only above 6 hours soaking periods [1]. At 1000˚C, the Alloy showed a marked and continuous decrease with increasing soaking periods. For the alloy, up to 8 hours soaking periods, volume fraction decreased only marginally up to 900˚C. For 10 hours soaking period, a marked and continuous decrease in the volume fraction was found.
The alloy was characterized for their corrosion behaviour. In fact, development of corrosion resistant white cast iron was one of the main aim of present work. Corrosion behaviour was studied with the help of weight loss method due to its reliability and reproducibility. Corrosion data have been summarized in Table IV.
A perusal of the Table IV revealed that: The corrosion resistance even in the as-cast state was satisfactory. The Alloy showed maximum corrosion rate. On heat treating from 800˚C, the corrosion rate matched with the corrosion rate of the as-cast the alloy. Corrosion rate decreased considerably for the alloy at and above 900˚C at all soaking periods.The maximum resistance against corrosion was at 10 hours soaking period and/or for 1000˚C heat treatment temperature. The surfaces of the specimens after corrosion testing were examined under a scanning microscope. No pitting or any other form of corrosion was revealed. Surface was attacked uniformly. Corrosion product was non-adherent.4
Figure 6: (900°C, 2 hrs.)
Figure 7: (900°C 10 hrs.)
Figure 8: (950°C, 2 hrs.)
Figure 9: (950°C, 6 hrs.)
Figure 10: (950°C, 10 hrs.)
Figure 11: (1000°C, 2 hrs.)
the needle formation is more after the incresing the tempreature
Discussions
Prior to analysing the results, it is essential to understand the behaviour of alloy under consideration.
A matrix having lesser amount of alloying elements has a natural tendency to transform to martensite and on air cooling there are fair chances that martensite will be present in the microstructure along with austenite[5].
An increase of either heat treating temperature for soaking period or of a soaking period for a heat treating temperature will not only coarse the dispersed carbides but also may promote the dissolution of dispersed carbides and may/ may not transform to martensite. Austenite precipitates dispersed carbides and austenite plus dispersed carbides are obtained. The transformed austenite will contain lesser alloy contents. Massive carbides will get discontinuous and their volume fraction will get reduced. Such a transformation will be accompanied by rejecting interstitial and substitutional solute elements. Massive carbides will get converted to other types of carbides. Interstitial and substitutional solute atoms available as a result of reduced volume fraction of massive carbides will go to austenite. This will give rise to formation of austenite having enhanced stability. Austenite having enhanced stability, on raising the heat treatment temperature or time, will precipitate dispersed carbides by lowering its stability. Dispersed carbides will increase with increase in temperature/time and finally will get coarsen. Another possibility also exists i.e. dispersed carbides may get dissolved into austenite at higher temperatures.6
The structural changes in the heat treated condition are responsible for variation of the hardness. Carbides get precipitated from the austenite during soaking and hence a reduction in the volume fraction of MCs and/or rounding off of the massive carbides observed. Martensite also gets decomposed on heating.
For the alloy at 800˚C, the matrix was comprised of martensite and austenite. Hardness is independent of soaking period for the alloy. The same trend has been shown by volume fraction of massive carbides. It justifies the hardness results. Hardness and massive carbide data follow the same trend i.e. both decrease relatively faster at soaking periods more than 4 hours and justify the results.
Figure 12: (1000°C, 6 hrs.)
Figure 13: (1000°C, 10 hrs.)
On heat treating at 850˚C, formation and coarsening of DCs and DSPs (needle / plate ) takes place. The coarsening tendency will be more at higher soaking periods. This explains the results obtained. Volume fraction of massive carbides follow the same trend as that of hardness.
In a similar manner, hardness, volume fraction and microstructure can be correlated for other heat treatment temperatures. Matrix is now austenitic.
The compressive strength data can be suitably interpreted in terms of initial volume fraction of massive carbides, the effect of heat treatment temperature and soaking period on the volume fraction of massive carbides, morphology of massive carbide as influenced by the heat treatment temperature and soaking period, formation of needle like carbides/ dispersed spherical carbides and influenced on their coarsening tendency/ interlinking either with massive carbides or amongst themselves, and morphology of these carbides as affected by the heat treatment temperature and soaking period. Matrix structure also play a significant role.
As-cast alloy (inherently brittle white cast irons) due to the presence of martensite as matrix, platy massive carbides and complex microstructural (highly heterogeneous system) exhibit poor compressive strength. Martensite is also present in alloy heat treated from lower heat treatment temperatures (say up to ≈ 900˚C). Role of needle like carbides/ dispersed spherical carbides is two-fold. These can either strengthen or embrittle the alloy depending on their favourable/adverse morphology. At higher temperatures/soaking periods, somewhat favourable effect is due to coarsening and reduction in their volume fraction. Similarly, reduced volume fraction of massive carbides and emergence of austenite as the major matrix structure at elevated temperatures enhance the compressive strength of the alloy. In fact, at higher temperatures, volume fraction of needle like carbides/ dispersed spherical carbides is of less importance. Here the strength will be governed by the volume fraction, distribution and morphology of massive carbides. Based on these reasoning, the compressive strength data can be explained with reasonably good accuracy. Fortunately, the data generated for the alloy is explainable on the basis of above stated facts.
For specimens heat treated from relatively lower temperatures, the corrosion rates were lower than the as-cast alloy inspite of the fact that the carbides in the form needles and DSP are present. It is a point of much satisfaction that this so called adversed morphology did not enhance the corrosion rate.
The SEM examination confirmed the absence of pitting and uniform dissolution of the material from the surface.
Conclusions
Though more detailed study and work is needed, it can be stated that corrosion resistant cast irons can be developed by using low cost indigenously available alloying elements. The microstructures having increased volume fraction of austenite were found to be useful from the corrosion resistance point of view. In fact, the corrosion rates attained on heat treating from higher temperatures in the experimental alloy are comparable or better than the ones attained in standard Ni-Resist (both flake & S.G. type) compositions being very extensively used under marine conditions. Morphology, amount and distribution of massive/ dispersed carbides not only influenced the hardness but also greatly influenced the response against corrosion.
References
- Sain P. K., Sharma C. P and Bhargava A. K. Microstructure Aspects of a Newly Developed, Low Cost, Corrosion-Resistant White Cast Iron. Metallurgical and Materials Transactions A. 2013.
- Metals Hand Book, 9th edition, ASM. Metals Park, Ohio. 1978;1(3-9):75-96.
- Angus H. T. Cast Iron- Physical and Engineering Properties. Butterworths, London. 1976;53:161-253 and 286-354.
- INCO. Engineering Properties and Applications of Ni-Hard Cast Irons. International Nickel Co., Inc., New York. 1978;2-15.
- Patwardhan A. K., Singh S. S and Jain N. C. Tool and Alloy Steels, October. 1987;369-377.
- Barton R. Special Cast Irons, Report No. 576. BCIRA Journal. 1960;8(6).
- Ohide T and Ohira G. British Foundry. 1983;76:Part:9.
- INCO. Ni-Resist Austenitic Cast Irons – Properties and Applications, International Nickel Co. Inc. 1965;1-21.
- Stefanescu D. M., Mitea D and Cracium S. AFS International Cast Metal Journal. 1976;1(2):19-26.
- Battelle B. L., Minkoff I And Mollard F. Editors The Metallurgy of Cast Irons, Proc. of 2nd. International Symposium, Geneva, Switzerland. 1974;29-31.
- Henon G. Properties and Uses of Irons Alloyed with Nickel, Copper and Chromium. Foundry Trade Journal. 1966;507-519.
- Bansal A. K., M. E. Dissertation. Malviya Regional Engineering College, Jaipur, University of Rajasthan. 1999.
- Srinivasan T., M. E. Dissertation, University of Roorkee, Roorkee. 1975.
- Sharma C. P., M. E. Dissertation, University of Roorkee, Roorkee. 1976.
- Sudan A. S., M. E. Dissertation, University of Roorkee, Roorkee. 1976.
- Singh S. S. Ph.D. Thesis, University of Roorkee, Roorkee. 1981.

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal