Development and Characterization of Drug-Loaded Self-Solid Nano-Emulsified Drug Delivery System for Treatment of Diabetes
Introduction
Diabetes mellitus, or simply diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the pancreas does not produce enough insulin, or because cells do not respond to the insulin produced. It had been observed, that the drugs (e.g., embelin and gliclazide), when administrated into diabetic rats caused a highly significant decline in the blood glycated hemoglobin. In addition to this, the serum glucose levels, and nitric oxide activity, with a concomitant increased the serum insulin level.1-2 Also, the diabetic mellitus has been treated orally with herbal remedies based on traditional medicines like Embelia ribes burm (Myrsinaceae).3 In this connection, the Gandhi et al., isolated embelin from Embelia ribes burm being a fruit extract and evaluated it for its potential role to regulate insulin resistance, altering b-cell dysfunction and modulating key markers involved in insulin sensitivity and glucose transport. High-fat diet and streptozotocin, (40mg/kg) were used to induced type 2 diabetes in rats.4 Other researchers have reported the antidiabetic, antidyslipidemic and antioxidant activity of E. ribes Burm in streptozotocin-induced diabetic rats, using gliclazide as the positive control drug.5 The gliclazide is a second generation sulphonylurea which acts as a hypoglycemic agent. It stimulates β-cells of the islet of Langerhans in the pancreas to release insulin. It also enhances peripheral insulin sensitivity. Overall, it potentiates insulin release and improves insulin dynamics. It is a lipophilic (log P=2.6) in nature and water-insoluble moiety belonging to Class II Drug (i.e., low solubility and high permeability).6
It is estimated that 40% of active drug substances are poorly water-soluble in nature. For the improvement of bioavailability of these drugs with such attributes presents one of the greatest challenges in drug formulations. Technological strategies like solid dispersions, cyclodextrin complex formation, and/or micronization are commonly used strategies.7 Recently, nanotechnology 8-9 has gained much attention and these strategies can be used to deliver drugs in these formulations via different delivery systems like nanofibers,10 nanoparticles,11 nanorods12 and nanoemulsions.13 Nanoemulsions in the form of self nano-emulsifying drug delivery system (SNEDDS) has gained much attention for enhancement of oral bioavailability with reduced dosage.14 The SNEDDS are ideally isotropic mixtures of oils, surfactants, and co-surfactant/co-solvents, which when introduced into aqueous phase emulsifies spontaneously to produce fine oil-in-water emulsions (or micro-emulsions/nanoemulsion) upon gentle and mild agitation.15 Currently, SNEDDS are developed and formulated by using surfactants having hydrophile-lipophile balance value less than 12.16 Furthermore, the oral delivery of solid dosage form of lipophilic drug compounds is usually hindered due to their high lipophilicity and low water solubility. Therefore, the oral route is the most preferred and desirable strategy for chronic drug therapy. Tremendous potent lipophilic drugs exhibit low oral bioavailability, low absorption, and low efficacy after in-vivo administration due to their poor aqueous solubility. When a least amount of the administered dosage reaches the pharmacological site of action. The remainder, later on causes toxicity and side-effects due to undesirable biodistribution. As far as biopharmaceutics classification system is concerned, only 34% of drugs belong to class I, while as remaining drugs belong to class II-IV (17% to class II, 39% to class III and 10% to class IV), respectively.17
SNEDDS have also attracted interest because they can improve the bioavailability of compounds that fall into class II (compounds are poorly water soluble and highly permeable) of the biopharmaceutics classification system. An additional advantage of SNEDDS over simple oily solutions is that they provide the large interfacial area for partitioning of the drug. For drugs subject to dissolution rate limited absorption, SNEDDS may offer an improvisation in both the rate of absorption and reproducibility of plasma concentration profiles.18 SNEDDS are usually formulated as the liquid and/or in liquid forms or they are encapsulated in soft gelatin capsules.19 However, to enhance the physical stability on long-term storage and to minimize the disadvantages associated with liquid SNEDDS such as incompatibility problems with capsule shells,19 low drug portability, drug leakage and precipitation, liquid SNEDDS can further be formulated into solid SNEDDS.
Materials and Methods
Embelin was purchased from Indofine chemical company, Inc, USA. Gliclazide sample was kindly gifted by Ranbaxy laboratories Gurgaon. Capmul® MCM was the generous gift of Abitec Corporation, NEUSILIN® UFL2 was provided by Fuji Chemicals-Gangwal Chemicals, Mumbai, India. Castor oil, olive oil, Propylene glycol, PEG 400, Tween 20, Tween 60, Tween 80 were purchased from Himedia Pvt. Ltd. Mumbai, India. Oleic acid and coconut oil were purchased from Loba Chemie Pvt. Ltd. Mumbai, India. Cremophor EL, Avicel® PH-200, Kolliphor® HS 15 were purchased from Sigma Aldrich, USA. Hydrochloric acid, potassium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, sodium hydroxide, Lactose monohydrate were purchased from Central drug house, Pvt. Ltd, New Delhi, India. Sodium Starch glycolate and aerosil® fumed silca were purchased from Ind. Swift Laboratories Pvt. Ltd, Himachal Pradesh, India. All chemicals were of analytical grade and directly used without any further purification.
Pseudo-ternary phase diagram was prepared by using chemix software 5 trial version having the triangular format which has three coordinates (Figure 1). Each coordinate represents one component of emulsion system viz. (1) Oil phase (2) Surfactant-cosurfactant SA-Cos ratio phase and (3) Aqueous phase. Each coordinate represents 0 to 100% concentration for each phase at the increment of 10%. Further, water titration method was used to construct pseudoternary phase diagrams, because this method is easy and scalable. In this study, blank (without drugs) SNEDDS were prepared to find the area of the particular component system. The surfactant was blended with co-surfactant in fixed weight ratios of (1:1, 2:1, 3:1, and 4:1) based on earlier reports.20 Aliquots of each surfactant and co-surfactant mixture (Smix) were then mixed with oil at ambient temperature. For each phase diagram, the ratio of oil to the Smix was varied as (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9 (v/v)). The water was added in a dropwise manner to each oil-Smix mixture under vigorous stirring conditions. After the equilibrium was achieved, the samples were visually checked and determined as being clear emulsions and/or as gels. Preparation of optimized embelin and gliclazide loaded liquid SNEDDS were prepared by using the ratio of oil-Smix and pseudo-ternary phase diagram was constructed according to the procedure mentioned elsewhere.20 Briefly, the embelin and gliclazide were dissolved in surfactant followed by addition of co-surfactant and oil in a beaker. The resultant mixtures were continuously agitated by magnetic stirrer at 400-800rpm and heated at 40°C to obtain a homogenous solution. Different batches of SNEDDS containing embelin and gliclazide were prepared and optimization was done on the basis of emulsification time, particle size, polydispersity index, and zeta potential.
Figure 1: Pseudoternary phase diagrams of Capmul® MCM (oil), Kolliphor® HS 15: PEG 400 (SA:Cos) and water system in surfactant to co-surfactant ratio of (a) 1:1, (b) 2:1, (c) 3:1 and (d) 4:1.
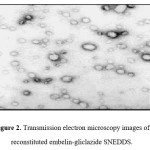
Figure 2: Transmission electron microscopy images of reconstituted embelin-gliclazide SNEDDS
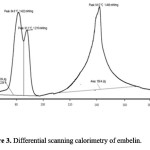
Figure 3: Differential scanning calorimetry of embelin.
The mean globule size and polydispersity index and zeta potential were determined using the particle size analyzer (Beckman Coulter Counter Delsa Nano C, USA, DLS 4C). The measurements of size and polydispersity index are based on light scattering, while zeta potential is based on the electrophoresis and electrical conductivity of the sample. The morphology of the SNEDDS was observed using transmission electron microscope (TEM) attached to a mega view II digital camera (H 7500, Hitachi, Tokyo, Japan). A drop of sample diluted with water was placed on a copper grid and the excess was drawn off using filter paper. For staining the nanoparticles, 1% of phosphotungstic acid was dropped on freshly nanoparticle loaded TEM copper grid. Further on, this TEM grid was dried in vacuum for future analyses. The image was magnified and focused on a layer of photographic film (Figure 2). The melting point of the drug was determined using Differential Scanning Calorimetry (DSC) to determine glass transitions, crystallinity, melting point, purity of materials, phase transitions, polymorphism, etc. The plain embelin, plain gliclazide and drug-loaded SNEDDS were first weighed in a crucible, crimped and fitted in the instrument (DSC 1 STAR system; Mettler Toledo-USA). The desired gas (i.e., nitrogen) was then purged and samples analyzed using the software. The DSC measurement was done with a sample weight of 3-5mg and the samples were heated from 0 to 250°C with a scanning speed of 10°C/min. The DSC thermographs of the drugs and SNEDDS are shown in (Figure 3-5). The optimized drug-loaded SNEDDS formulations were subjected to stability study for a period of two months at room temperature and in refrigeration conditions. After two months of storage, samples withdrawn were reconstituted into nanoemulsion and were subjected to test for drug content, particle size, and zeta potential. The drug release studies were conducted using liquid SNEDDS (equivalent to 3mg gliclazide and 9mg embelin) filled in size ‘00’ hard gelatin capsules. The in-vitro release profiles were studied using USP Dissolution Test apparatus II at 37°C with a rotation speed of 50rpm in 500ml phosphate buffer. The aliquots containing 1ml of samples were withdrawn at predetermined time intervals (i.e., after 1hrs) and filtered through 0.45μm filter. The withdrawn volume was replenished immediately with the same volume of fresh medium in order to maintain sink conditions. The amount of embelin and gliclazide released in the dissolution medium was determined using UV Spectrophotometer at 226.98 and 292.75nm. The blank SNEDDS (without drug) was processed similarly and used as a reference to avoid interference from the formulation components if any. The mean of at least three determinations was used to calculate the drug release.21 The in-vivo studies using Wistar rats (either sex) weighing about 180-220gm were procured from disease-free animal house LLUVAS, Hisar (India) and kept in the central animal house of I.S.F. College of Pharmacy, Moga (Reg. No. 816/PO/a/04/Control and supervision of experiments on animals, CPCSEA). The protocol of the experiment (IAEC/CPCSEA /2014/197) was approved by institutional animal ethics committee and the experiments were conducted in accordance with guidelines as per CPCSEA. Diabetes was induced by a single intraperitoneal injection of freshly prepared streptozotocin (45mg/kg body weight) in 0.1M citrate buffer (pH 4.5) to overnight fasted rats. Streptozotocin injected rats were allowed to drink 20% glucose solution for 24hrs to prevent streptozotocin-induced hypoglycaemic mortality.22 The development of diabetes was confirmed after 1 week of streptozotocin injection, the animals with fasting blood glucose level more than 200mg/dl were selected for the present study.23 The animals were divided into six groups (n=6) and single dose of each prepared combination was administered orally. The blood samples were withdrawn from the tail after 1hrs of the study. Drug treatment was started after one week of streptozotocin administration. The animals were fasted overnight before starting the experiment. The rats were randomly assigned to five different groups (n=6). The control and diabetic control group received saline solution; standard groups received pure embelin at a dose of 30mg/kg/day and gliclazide at a dose of 10mg/kg/day in suspension form via oral canula. Furthermore, the treated groups received embelin (30mg/kg/day)+gliclazide (10mg/kg/day) loaded SNEDDS formulations. The blood was withdrawn from the tail vein and estimated for blood serum glucose level, serum cholesterol level, and serum triglyceride levels as per the approved methods. The statistical analysis involving two groups was evaluated by means of two-way ANOVA followed by Bonferroni post-test whereas two-way ANOVA followed by Tukey post-test was used for column analysis and P values (P<0.001 and P<0.05) were considered to be significant.
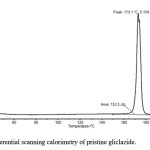
Figure 4: Differential scanning calorimetry of pristine gliclazide
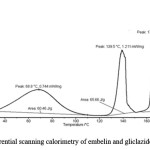
Figure 5: Differential scanning calorimetry of embelin and gliclazide SE pellets.
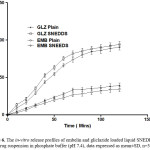
Figure 6: The in-vitro release profiles of embelin and gliclazide loaded liquid SNEDDS and plain drug suspension in phosphate buffer (pH 7.4), data expressed as mean±SD, n=3.
Figure 7: Effect on serum glucose levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control, cP<0.05 versus EMB (30mg/kg)]
Result and Discussions
Self-emulsifying systems form a fine oil-water emulsion with only gentle agitation, upon their introduction into aqueous media. Surfactant and co-surfactant get preferentially adsorbed at the interface, reducing the interfacial energy as well as providing a mechanical barrier to coalescence. The decrease in the free-energy required for the emulsion formation consequently improves the thermodynamic stability of the emulsion formulation.24 Therefore, the selection of oil or surfactant, and the mixing ratio of oil to Smix plays a significant role in the formation of the emulsion. Screening of surfactant: co-surfactant ratio was done on the basis of pseudoternary phase diagram using water titration method. In this regard, the (Figure 1) shows the screening of different ratios of surfactant: co-surfactant and pseudoternary phase diagrams prepared for different ratios of surfactant and cosurfactant S: CoS (i.e., from 1:1, 1:2, 1:3 and 1:4).
On the basis of this pseudoternary phase diagram study, the optimized ratio of surfactant and co-surfactant i.e., Kolliphor® HS 15: PEG 400 was 2:1. The pseudoternary diagrams showed that with the increase in the surfactant concentration there is increase in the emulsifying region. The selection was totally on the basis of the region of emulsification i.e. more the emulsification region better will be the ratio. Selected ratio i.e., 2:1 amongst other ratios provides the rapid emulsification and reduced globule size of the emulsion. The optimization of the liquid SNEDDS formulation was achieved from above ternary phase diagrams, S: CoS (2:1) give maximum area, so it should the give stable emulsion. Based on the observations in the phase diagram, different batches of SNEDDS containing embelin and gliclazide were prepared by increasing the concentration of the oil and diluting in a fixed volume of water. The optimization parameters for the respected batches were emulsification time, % transmittance, particle size, polydispersity index, zeta potential and precipitation of the drug. The dose of the drugs and ratio of surfactant: co-surfactant was kept constant. With the increase in the oil concentration the S:CoS concentration was decreased simultaneously because of the fixed formulation size.
The concentration of drugs was fixed on the basis of percentage reduction in blood glucose level on five groups of Wistar rats (n=6). The optimization chart showed an increase in the ratio of the oil phase (Capmul® MCM) resulted in a proportional increase in particle size, because of the simultaneous decrease in the S/CoS proportion. Increasing the S/S-Co ratio led to a decrease in mean droplet size. This could be attributed to an increased surfactant proportion relative to co-surfactant. It is well known that the addition of surfactants to the emulsion systems causes the interfacial film to stabilize and condense, while the addition of co-surfactant causes the film to expand, thus, the relative proportion of surfactant to co-surfactant has varied effects on the droplet size. Similarly, there is a decrease in % transmittance value with increase in the concentration of the oil due to increase in globule size of the emulsion formed. Negative zeta potential value of all the respected batches shows the presence of fatty acids in the oil phase. For oral emulsion preparations, dilatability is a very important parameter to confirm that the drug should not precipitate out on dilution in GIT. Formulation (F9) was the selected one on the basis of emulsification time, no precipitation, zeta-potential, transmittance and polydispersity index.
Figure 8: Effect on weight in Wistar rats by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control, cP<0.05 versus EMB (30mg/kg)]
Figure 9: Effect on serum cholesterol levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control].
Figure 10: Effect on serum triglyceride levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control].
It is necessary to assess zeta potential of the SNEDDS as it can identify the charge of oil globules in the emulsion.25 The increase in electrostatic repulsive forces between the globules prevents the coalescence of emulsion. On the contrary, a decrease in electrostatic repulsive forces can cause phase separation. The mean zeta potential of the emulsion obtained by diluting embelin loaded SNEDDS with distilled water was -34.35 mV. The charge on the oil globules is negative due to free fatty acid present in the oil phase.25 The developed SNEDDS can be filled in hard gelatin capsules by commercial liquid filling equipments.26 The morphology of the reconstituted EMB- gliclazide-SNEDDS formulation was observed using TEM (Figure 2). It reveals discrete, spherical oil globules less than 200nm in size, which was in accordance with the findings using Beckman coulter counter. As the oil globules were discrete and non-aggregated, we can state that the nanoemulsion was physically stable.27 The melting point of gliclazide was found to be 173.3°C. The DSC of embelin exhibited two exothermic peaks at 84.80°C and 141.2°C resulting from loss of water and melting point peak. Figure 3 to 5 shows the DSC results of embelin, gliclazide, and embelin-gliclazide pellets, as observed after centrifugation. The peak that was obtained in thermo gram gives the characteristic melting point of that molecule. From the Figure 5, it is clear that the SNEDDS of gliclazide and embelin all ingredients indicate no chemical interaction and/or incompatibility between drugs and the other ingredients. However, it may be observed that peaks of embelin are missing for instance, peak at 84.80oC, this may because water and other ingredients. The reduction in peak intensity of drugs is a consequence of less overall drug content present in SE pellets as compared to drugs alone.28
The optimized batch of SNEDDS formulation was kept for stability at refrigerated and ambient temperature. Stability study was designed to observe the resistance of nanoemulsion from coalescence and phase separation when a critical gravitational force is applied (7500 to 10,000 rpm for 10 minutes). This test also verifies the nature of the nanoemulsion, monophasic or biphasic system. No phase separation was observed by centrifugation even at 10,000 rpm for 10 minutes.
The in-vitro drug release of embelin and gliclazide loaded liquid SNEDDS in comparison to the plain drug suspension in phosphate buffer pH 7.4 is shown in Figure 6. Percentage cumulative release was 92.23±1.22% for embelin and above 95.36±3.43% for gliclazide loaded liquid SNEDDS. Moreover, it was only 26.82±2.14% in case of plain drug suspension of embelin and 33.19±1.76% for the plain drug suspension of gliclazide after 70 mins. The significant increase in the rate of release of embelin and gliclazide from SNEDDS compared to pure drug suspension can be attributed to its quick dispersibility and ability to keep drug in the solubilized state.
The in-vivo studies carried on the optimized formulation demonstrated a significant reduction in blood glucose level (Figure 7). The streptozotocin treated group causes a significant increase in serum glucose level as compared to vehicle-treated group. The embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups show a significant decrease in serum glucose level as compared to streptozotocin treated group and other combinations (P<0.05). Embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS making it an effective combination in reversing streptozotocin induced hyperglycaemia. However, the group receiving a combination of the free drugs i.e., (embelin (30mg/kg), gliclazide (10mg/kg)) along with gliclazide (10mg/kg) loaded SNEDDS was more effective in reducing serum glucose levels compared to the streptozotocin treated group (P<0.05). The effect of these formulation combinations was also evaluated on the body weight of the animals as shown in Figure 8. It was observed that streptozotocin treated group cause a significant decrease in body weight as compared to vehicle-treated group on 7th, 14th and 21st day. Embelin (30mg/kg), gliclazide (10mg/kg), and Embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups present a significant elevation in body weight as compared to streptozotocin treated group on 7th, 14th and 21st day (P<0.05). However, gliclazide (10mg/kg), and Embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups show the significant difference as compared to embelin (30mg/kg) treated group on 7th, 14th and 21st days, respectively. Furthermore, streptozotocin administration produced the significant elevation in serum cholesterol level as compared to vehicle-treated group in Wistar rats as presented at (Figure 9). Embelin (30mg/kg), gliclazide (10mg/kg), and Embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups significantly attenuated streptozotocin induced elevation in serum cholesterol level (P<0.05). However, gliclazide (10mg/kg), and embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated group indicate the significant difference as compared to embelin (30mg/kg) treated group. Similarly, streptozotocin administration produced significant elevation in serum triglyceride levels as compared to vehicle-treated group in Wistar rats (Figure 10). Embelin (30mg/kg), gliclazide (10mg/kg), and embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups significantly attenuated streptozotocin induced elevation in serum triglyceride level (P<0.05). However, gliclazide (10mg/kg), and embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS treated groups show a significant difference as compared to embelin (30mg/kg) treated group.
Conclusion
The optimized formulations of the embelin and gliclazide liquid SNEDDS was successfully developed in our study. The percent potency of embelin and gliclazide in liquid SNEDDS was significantly increased when compared to pure drugs. Thus, the present study proved the significant potentials of liquid SNEDDS in enhancing the dissolution rate and solubility of embelin and gliclazide. Embelin (30mg/kg)+gliclazide (10mg/kg) loaded SNEDDS was selected as optimized formulation as it was found to be the most effective combination in reversing streptozotocin induced hyperglycaemia as compared to the pure embelin and gliclazide. The enhanced results of optimized formulation were produced because embelin and gliclazide administration to diabetic rats cause a highly significant decline in the blood glycated hemoglobin, serum glucose levels, and nitric oxide activity. This can be attributed to a concomitant increase in the serum insulin level and regeneration of islet cells by embelin treatment.
Acknowledgement
This work was supported by research grants from ISF College of Pharmacy, Ghal Kalan, Ferozpur G.T. Road, Moga, Punjab. Also, the partial financial support to authors was provided by DST Nano Mission sponsored project (SR/NM/NM-1038/2016) and Science and Engineering Research Board (SERB) research grants (ECR/2016/001429).
Funding Source
This work was supported by research grants from ISF College of Pharmacy, Ghal Kalan, Ferozpur G.T. Road, Moga, Punjab.
Conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article
References
- Mahendran S., Badami S., Maithili V. Evaluation of antidiabetic effect of embelin from Embelia ribes in alloxan induced diabetes in rats. Biomedicine & Preventive Nutrition. 2011;1(1):25-31.
CrossRef
- Guerra S. D., Grupillo M., Masini M., Lupi R,, Bugliani M.,Torri S., Boggi U.,Chiaro M. D., Vistoli F. Mosca F. Gliclazide protects human islet beta‐cells from apoptosis induced by intermittent high glucose. Diabetes/metabolism research and reviews. 2007;23(3):234-238.
CrossRef
- Bhandari U., Ansari M. N. Antihyperglycaemic activity of aqueous extract of Embelia ribes Burm in streptozotocin-induced diabetic rats. 2008.
CrossRef
- Gandhi G. R., Stalin A., Balakrishna K., Ignacimuthu S., Paulraj M. G., Vishal R. Insulin sensitization via partial agonism of PPARγ and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(1):2243-2255.
CrossRef
- Bhandari U., Jain N., Ansari M., Pillai K. Beneficial effect of< i> Embelia ribes</i> ethanolic extract on blood pressure and glycosylated hemoglobin in streptozotocin-induced diabetes in rats. Fitoterapia. 2008; 79(5):351-355.
CrossRef
- Palmer K. J., Brogden R. N. Gliclazide. Drugs. 1993;46(1):92-125.
CrossRef
- Khadka P., Ro J., Kim H., Kim I., Kim J. T., Kim H., Cho J. M., Yun G., Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian journal of pharmaceutical sciences. 2014;9(6):304-316.
CrossRef
- Sheikh F. A. Barakat N. A. M. Kim B. S. Aryal S. Khil M. S. Kim H. Y. Self-assembled amphiphilic polyhedral oligosilsesquioxane (POSS) grafted poly(vinyl alcohol) (PVA) nanoparticles. Materials Science and Engineering: C. 2009;29(3):869-876
CrossRef
- Wani S., Sofi H. S., Sheikh F. A., Shivashankar S. Majeed S. ZnGa2O4 Nanophosphors: Rapid Synthesis, Characterization and Luminescence Properties. Material Science Research India. 2017;14(2):116-122.
CrossRef
- Wani S., Sofi H. S., Majeed S., Sheikh F. A. Recent Trends in Chitosan Nanofibers: From Tissue-Engineering to Environmental Importance: A Review. Material Science Research India. 2017;14(2):89-99.
CrossRef
- Anvekar T. S., Chari V. R., Kadam H. Green Synthesis of ZnO Nanoparticles, its Characterization and Application. Material Science Research India. 2017;14(2):153-157.
CrossRef
- Zhang C., Li C., Huang S., Hou Z., Cheng Z., Yang P., Peng C., Lin J. Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery. Biomaterials. 2010;31(12):3374-3383.
CrossRef
- Lovelyn C., Attama A. A. Current state of nanoemulsions in drug delivery. Journal of Biomaterials and Nanobiotechnology. 2011;2(05);626.
CrossRef
- Kohli K., Chopra S., Dhar D.,Arora S.,Khar R. K. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug discovery today. 2010;15(21-22):958-965.
CrossRef
- Nazzal S., Smalyukh I.,Lavrentovich O., Khan M. A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. International journal of pharmaceutics. 2002;235(1):247-265.
CrossRef
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X., Design and optimization of a new self-nanoemulsifying drug delivery system. Journal of colloid and interface science. 2009;330(2):443-448.
CrossRef
- Lindenberg M., Kopp S., Dressman J. B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58(2);265-278.
CrossRef
- Shukla J. B.,Patel S. J. Formulation and evaluation of self micro emulsifying system of candesartan cilexetil. Int. J. Pharm. Pharm. Sci. 2010;2:(4):143-146.
CrossRef
- Yi T.,Wan J., Xu H., Yang X. A new solid self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs. European Journal of Pharmaceutics and Biopharmaceutics 2008;70(2):439-444.
CrossRef
- Kallakunta V. R., Bandari S., Jukanti R., Veerareddy P. R. Oral self emulsifying powder of lercanidipine hydrochloride: formulation and evaluation. Powder Technology. 2012;221:375-382.
CrossRef
- Basalious E. B., Shawky N., Badr-Eldin S. M. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. International journal of pharmaceutics. 2010;391(1);203-211.
CrossRef
- Al-Numair K. S., Pugalendi K. V. Effect of 3-hydroxymethyl xylitol on hepatic and renal functional markers and protein levels in streptozotocin-diabetic rats. African Journal of Biochemistry Research. 2009:3(5), 198-204.
CrossRef
- Shanmugasundaram E., Gopinath K. L.,Shanmugasundaram K. R., Rajendran V. Possible regeneration of the islets of langerhans in streptozotocin-diabetic rats given gymnema sylvestre leaf extracts. Journal of ethnopharmacology. 1990;30(3):265-279.
CrossRef
- Patel P.,Chaulang G.,Akolkotkar A., Mutha S., Hardikar S., Bhosale A. Self emulsifying drug delivery system: a review. Research Journal of Pharmacy and Technology. 2008:1(4):313-323.
CrossRef
- Yoon R. H., Yordan J. L. Zeta-potential measurements on microbubbles generated using various surfactants. Journal of Colloid and Interface Science. 1986;113(2):430-438.
CrossRef
- Gershanik T.,Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:(1):179-188.
CrossRef
- Hauss D. J., Fogal S. E., Ficorilli J. V., Price C. A., Roy T., Jayaraj A. A., Keirns J. J. Lipid‐based delivery systems for improving the bioavailability and lymphatic transport of a poorly water‐soluble LTB4 inhibitor. Journal of pharmaceutical sciences. 1998;87(2):164-169.
CrossRef
- Gupta S., Chavhan S., Sawant K. K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids and Surfaces A, Physicochemical and Engineering Aspects. 2011;392(1):145-155.
CrossRef
- Taha E. I., Al-Saidan S., Samy A. M., Khan M. A. Preparation and in vitro characterization of self-nanoemulsified drug delivery system (SNEDDS) of all-trans-retinol acetate. International journal of pharmaceutics. 2004;285(1):109-119.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Taha Umair Wani1
, Taha Umair Wani1 , Neeraj Mishra1
, Neeraj Mishra1 , Hasham S. Sofi2
, Hasham S. Sofi2 , Roqia Ashraf2
, Roqia Ashraf2 and Faheem A. Sheikh2*
and Faheem A. Sheikh2*
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal


![Figure 7. Effect on serum glucose levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control, cP<0.05 versus EMB (30mg/kg)]](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Dev_Muz_Fig7-150x150.jpg)
![Figure 8. Effect on weight in Wistar rats by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control, cP<0.05 versus EMB (30mg/kg)]](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Dev_Muz_Fig8-150x150.jpg)
![Figure 9. Effect on serum cholesterol levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control].](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Dev_Muz_Fig9-150x150.jpg)
![Figure 10. Effect on serum triglyceride levels by embelin, gliclazide and embelin+gliclazide-loaded SNEDDS formulation. [aP<0.05 versus normal control, bP<0.05 versus diabetic control].](http://www.materialsciencejournal.org/wp-content/uploads/2018/04/Vol15_No1_Dev_Muz_Fig10-150x150.jpg)

