Banti Ganguly
Department of Chemistry, BBM College, Agartala, Tripura
Corresponding Author E-mail: mamon.0123@gmail.com
DOI : http://dx.doi.org/10.13005/msri/160304
Article Publishing History
Article Received on : 28-11-2019
Article Accepted on : 21-12-2018
Article Published : 21 Dec 2019
Plagiarism Check: Yes
Reviewed by: Bharath V
Second Review by: SHANIGARAPU SHARADA
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
In this short review paper we have discussed about the green nanoparticles synthesis, types, application, mechanism of synthesis and bioactivities. The green nanoparticles prove to provide clean, nontoxic materials which are important in our life survivals. This short review paper was concern with AgNPs and the reactions criterion of which such nanoparticles being prepared confined a pronounced impact on their dimension, shapes and applications.
KEYWORDS:
Green Nanoparticles; Synthesis; Bioactivities
Copy the following to cite this article:
Ganguly B. Short Review on Green Silver Nanoparticles and its Bioactivities. Mat. Sci. Res. India; 16 (3).
|
Copy the following to cite this URL:
Ganguly B. Short Review on Green Silver Nanoparticles and its Bioactivities. Mat. Sci. Res. India; 16 (3). Available from: https://bit.ly/2Z9Ij5c
|
Introduction
Nanoscience and nanotechnology is a promising and desirable field in every subject matters including medical, environmental and allied course. Nanoparticles has dimension in the range 1-100nm. The green nanoparticles prepared have varied purposes such as sensing, dye degradation, cosmetics, drug delivery, food industry, antioxidant etc.1-6
The synthesis of nanoparticles (NPs) can be executed using different methods, including physical, chemical, and biological techniques.7 The NP synthesis by predictable physical and chemical techniques hold the risk of toxicity and environmental pollution as they discharge toxic derivative, which are potentially perilous to the environment.8 In addition to it, the NPs synthesized by such hazardous methods are unhealthy for the medical field due to the health-related apprehension, particularly in clinical applications.9
The current years have witnessed a mounting interest in the nanosynthesis of environment-friendly particles without concerning the production of toxic by-products as part of the synthesis process.10 The tool used for the green synthesis nanoparticles involves clean, toxic free, less energy and quick response.
AgNPs has received a quite a lot consideration as disinfectant and antimicrobial agent.11 For the concern of community and environment has motivated and impelled us to work in the plant mediated green synthesis and bioactivities of AgNPs.12-13
Different types of Nanoparticles
According to the literature survey14 there are different types of nanoparticles. On this basis, the schematic diagram has been represented as in Scheme 1.
Scheme 1: Diagram for Nanoparticles Category
Different Synthetic Process of Nanoparticles
Nanoparticles production includes bottom-up and top-down approaches.15 Top-down is steady while the bottom down method undergoes self assembly of atomic size particles to form nanosize particles. Table 1 shows different methods for nanoparticles synthesis using physical, chemical and green processes.
Table 1: Methods of Nanoparticle Synthesis
|
Physical
|
Chemical
|
Green/biological
|
|
Mechanical methods
|
Co-precipitation
|
Plant extracts-mediated
|
|
Vapor deposition
|
Sol-gel
|
Microbial culture-mediated
|
|
Sputter deposition
|
Microemulsions
|
Agricultural waste-mediated
|
|
Electric deposition
|
–
|
Enzymes-mediated
|
|
Ion beam method
|
–
|
Plant- Based Nanopartlces
It has been reported already in the literature the plant resources based green synthesis of a number of metal NPs.16-17 Antimicrobial, anticancer, antidiabetic, anti-inflammatory, antioxidant etc are the different action which plany based NPs posses.18 The phytochemicals such as flavonids, phenols, sugar, terpenoids, proteins etc present in the extract were involved in the capping, stabilization and bioreduction of metal ions.19
Green Synthesis of Nanoparticles using Plants Extract
Various parts of the plants have been used as fresh or dry material such as the fruit, leaf, peel, petal, and shoot for preparation of the plant extract. The NPs were extracted by soaking the plant matters in green solvents by stirring and followed by filtration and centrifugation process and this filtrate laden with reducing and capping agents were then used for the bioreduction of metallic ions. The benefit of using dried plant is that it has a long shelf life at room temperature, but it is essential to store the fresh plant at −20°C to avoid any worsening. In addition, the use of dry plant material ensures the abolition of effects of seasonal variations leading to disparity in plant constituents.20 Primary factors like temperature, pH, concentration of extract of plant materials and metal ions leverage the shape and size of the prepared NPs.21 Generally, the room temperature is the pre-requisite for the synthesis of metal NPs. The easy and flexible circumstances of reaction provide the plant based synthesis of metal NPs to be more appropriate for the huge production rather than any other based NPs synthesis.22
Green Synthesis of Silver NPs
The prime requisite of green synthesis of AgNPs is silver metal ion solution and a reducing biological agent. Explicitly, reducing agents or other component present in the cells activate as stabilizing and capping agents, so there is no necessity of accumulation of capping and stabilizing agents apparently.
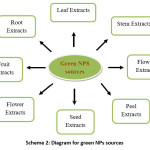
Many researchers have worked on the green synthesis of AgNPs using plenty of leaf, root, stem, flowers, peels, fruits extract and have given a notable application in different areas. An author used Eugenia jambolana leaf extract23 for the synthesis of AgNP. Another author24 used the bark extract of Saraca asoca for assuring the presence of hydroxylamine and carboxyl group in the extract. Leaves of Rhynchotechum ellipticum25 and Fruit extracts of Malus domestica26 were also utilized for the synthesis of AgNPs by researchers to enhance the presence of polyphenols, flavonoids, terpenoids etc.
Scheme 2: Diagram for green NPs sources
Mechanism of AgNP synthesis
The green method for the synthesis of AgNPs has immense importance for the existence of plenty of chemicals like carbohydrate, fat, proteins, phenols, flavinoids, terpenoids etc as these chemicals are proficient of electron donating for the reduction of Ag+ ions to Ag0. For the reduction of Ag+ ion the extract of plant materials plays an important role.
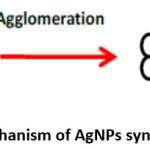
The AgNPs are synthesized by green method because of the existence of huge number of chemicals present viz. carbohydrate, fat, proteins, enzymes & coenzymes, phenols flavanoids, terpenoids, alkaloids, gum etc. These organic chemicals are competent of donating electron for the reduction of Ag+ ions to Ag0. Scheme 3 shows the reduction of Ag+ ion to Ag0, then agglomeration of the Ag0 followed by stabilization process to form a AgNPs.27
Scheme 3: Mechanism of AgNPs Synthesis
Analysis of SEM and TEM Micrographs
The morphology of the cell with the action of AgNPs can be examined by scanning electron microscopy and Transmission electron microscopy. The workers observed and also indicated the clear changes in the cell shape and perforation and it has been used as antimicrobial action.28
Bioactivities
Antibacterial Activity of AgNP
It has been known for so long that silver is toxic to bacteria being acting as antibiotic. Owing to the wide range of applications including pharmacological, agricultural, medicine and textiles, the AgNPs showed a varied interest for many researchers.
Antifungal Activity of AgNPs
Various pathogenic activities have given rise to insidious infections and bacterial effects also raised to an alarming rate and thus AgNPs finds its way as important and efficient antifungal agent
Antiviral Activity of AgNPs
AgNPs proves to provide significant antiviral activities. With smaller AgNPs the antiviral activity generally becomes stronger.
Anticancer Activities
Cancer is a life frightening disease and might sometime lead to death. The principal requirement at present is the synthesis of competent anticancer agent which can provide healing of such cancerous cell. Hence the synthesis of anticancer agents is of the prime necessity.29
Wound Dressing
In many a case Nanotechnology contributed in the chapter of wound healing as for anti-inflammatory and anti-microbial activities. Researchers and Scientist have worked hard enough for find out some interesting properties of AgNPs in wound healings. NP of size 22 nm proves to provide healing power which has been synthesized using cotton fabric.30 AgNPs impregnated into bacterial cellulose also showcased in wound healing process.31
Conclusion
This review paper has been concluded in the way that green nanoparticles proves to show huge application over chemical and physical processes. The AgNPs synthesized using the biological methods is said to be clean, non toxic, economic and green. The morphological analysis using spectroscopy and microscopy endow with the shapes and size of the synthesized NPs and also the surface topology. AgNPs also provides immense application in biosensor, medical applicability, catalyst etc. The future scope of this research includes in the drug delivery system as nano carriers. Even in dentistry, the AgNPs may be utilized in dental instruments and swathes and also can be incorporated in orthodontic glues.
Acknowledgment
The author is thankful to the Department of Chemistry, Tripura University and Bir Bikram Memorial College for constant support and encouragement.
Funding Source
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
There are no conflicts of interest.
References
- Lu, R., Yang, D., Cui, D., Wang, Z. and Guo, L. (2012) International Journal of Nanomedicine, 7, 2101-2107
- Chen, Q., Jiang, H., Ye, H., Li, J. and Huang, J. (2014) Journal of Carbohydrate Chemistry, 33, 298-312.
- El-Rafie, H.M., El-Rafie, M.H. and Zahran, M.K. (2013) Carbohydrate Polymers, 96, 403-410.
- Ajitha, A., Reddy, Y.A.K. and Reddy, P.S. (2014) Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 121, 164-172
- Saratale, R.G., Karuppusamy, I., Saratale, G.D., Pugazhendhi, A., Kumar, G., Park, Y. (2018) Colloids and Surfaces B: Biointerfaces, 170, 20-35
- Lopes, CR.B., Courrol, L.C. (2018) Journal of Luminescence, 99, 183-187.
- Khan, Z., Al-Thabaiti, S.A., Obaid, A.Y. and Al-Youbi, A.O. (2011) Colloids and Surfaces B: Biointerfaces, 82, 513-517.
- Dhand,V., Soumya, L., Bharadwaj, S., Chakra, S., Bhatt, D., Sreedhar, B. (2016) Mater Sci Eng C. 58:36–43.
- Moteriya, P., Chanda, S., (2017) Artif Cells Nanomed Biotechnol., 45, 1556–1567.
- Sharma, K., Kaushik, S., Jyoti, A., (2016) J Pharm Sci Res, 8, 313-321.
- McGillicuddy, E., Murray, I., Kavanagh, S., (2017) Sci Total Environ.575, 231–246.
- Morones, J.R., Elechiguerra, J.L., Camacho, A., (2005) Nanotechnology, 16, 2346-2356.
- Vijayan, S.R., Santhiyagu, P., Ramasamy, R.,(2016) Enzyme MicrobTechnol. 95, 45–57.
- Arunachalam, K.D., Annamalai, S.K., Hari, S., (2013) Int J Nanomedicine. 8, 1307–1315.
- Arole, V., Munde, S., (2014) J Adv Appl Sci Technol, 1, 89–93.
- 16. Hubbuch, J., (2016) poster presented at: posters-at-capitol wisteren ken-
- pucky university; March 3.
- Kumar, P.V., Kala, S.M.J., (2018) Int J Recent Res Aspects. 608-612.
- Fahimirad, S., Ajalloueian, F., Ghorbanpour, M., (2019) Ecotoxicol Environ Saf 168,260-78.
- Khodadadi, B., Bordbar, M., Nasrollahzadeh, M., (2017) J Colloid Interface Sci 490, 1-10.
- Altemimi, A., Lakhssass,i N., Baharlouei, A., Watson, DG., Lightfoot, D.A., (2017) Plants (Basel) 6:42-49.
- Vijayaraghavanab, K., Ashok kumar T., (2017) J Environ Chem Eng 5, 4866-4883.
- Ahmed, S., Ahmad, M., Swami, B.L., Ikram, S., (2016) J Adv Res 7, 17-28.
- Gomathi, S., Firdous, J., Bharathi, V., (2017) Int J Res Pharm Sci. 8, 383–387.
- Banerjee, P., Nath, D., (2015) Nanosci Technol. 2, 1–14.
- Hazarika, D, Phukan, A., Saikia, E., Chetia, B., (2014) Int J Pharm Pharm Sci., 6, 672–674.
- Mallikarjuna, K., Sushma, N.J., Narasimha, G., Manoj, L., Raju, B.D.P., (2014) Arabian J Chem., 7, 1099–1103.
- Jha, A.K., Prasad, K., Prasad, K. and Kulkarni, A.R. (2009) Colloids and Surfaces B: Biointerfaces, 73, 219-223.
- Zhang, M., Zhang, K., Gusseme, B.D., Verstraete, W. , Field, R. (2014) J. Bioadhesion and Biofilm Research, 30, 347-357.
- Sánchez-Navarro, M., Ruiz-Torres, C.A., Niño-Martínez, N., (2018) Bioinorg Chem Appl. 2018-8.
- Zodrow , K., Brunet, L., Mahendra, S., (2009) Water Res. 43,715–723.
- Hebeish, A., El-Rafie, M., El-Sheikh, M., Seleem, A.A., El-Naggar, M.E. (2014) Int J Biol Macromol. 65,509–515.
Views: 727
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal