CNT-Ni-Co-O based composite for Supercapacitor applications by Cyclic Voltametry analysis: A Short Quick Glimpse
Soumya Mukherjee
Department of Metallurgical Engineering, School of Mines & Metallurgy, Kazi Nazrul University, 713340, Asansol, India
Corresponding Author Email: smmukherjee3@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170104
Article Publishing History
Article Received on : 31-01-2020
Article Accepted on : 01-04-2020
Article Published : 01 Apr 2020
Plagiarism Check: Yes
Reviewed by: Dr. Karthikeyan Chelladurai
Second Review by: Dr. Bharat Makwana
Final Approval by: Jit Satyabrata
Article Metrics
ABSTRACT:
CNT based material are of vital importance in modern technology for their superior physical and chemical properties. In recent times, materials development for energy applications is focused for improvement of battery, capacitors, and electrodes for enhanced efficiency. High performance Supercapacitors with high energy densities are at the leading edge for renewable energy engineering device sector. CNT based Ni-Co-O material is of keen interest due to its possible applications as supercapacitors, electrocatalyst for metal/air battery and others. The hybrid material synthesis, morphological and electrochemical features are vital to evaluate the material performances for energy applications. Electrical studies are also important to evaluate the properties required for device applications. CNT is used as electrode material for electrochemical storage due to superior chemical stability, low mass density, low resistivity and large surface area. CNT replaces activated carbon material as supercapacitor due to improper balance between enhanced surface area and mesoporosity thus limiting electrolytic accessibility and capacitance. In the present article a brief review is stressed forward for the development of CNT-Ni-Co-O based hybrid material for supercapacitor high energy density applications.
KEYWORDS:
Ni-Co-O; CNT; Supercapacitor; Cyclic voltammetry
Copy the following to cite this article:
Mukherjee S. CNT-Ni-Co-O based composite for Supercapacitor applications by Cyclic Voltametry analysis: A Short Quick Glimpse. Mat. Sci. Res. India; 17(1).
|
Copy the following to cite this URL:
Mukherjee S. CNT-Ni-Co-O based composite for Supercapacitor applications by Cyclic Voltametry analysis: A Short Quick Glimpse. Mat. Sci. Res. India; 17(1). Available from: https://bit.ly/2ULm8QY
|
Introduction
With progress of technology focus is always laid on energy utilization, demand and also on sustainability for future. To sustain the ever growing demand of energy more stress is on development of materials for high performance electrodes, energy storage. High energy densities, high power delivery, low cost, low operating temperature make these materials suitable for energy applications. In recent years researchers are focussing on supercapacitors or electrochemical capacitors for high energy density storage. Supercapacitors are characterized by high power densities, fast charging/discharging capability and outstanding cyclic stability. Power density is the most important criteria for supercapacitance but energy density is smaller than secondary battery. This particular is one of the major hindrances towards its device applications.1,2,3 Electrochemical supercapacitor consists of two electrodes, electrolyte and a separator that isolates the two electrodes electrically. Based on the energy storage mechanism two types of supercapacitors are classified; Electrical double layer capacitor (EDLC) mainly consist of carbonaceous compounds and pseudocapacitors which rare transitional metal oxides/hydroxides, conducting polymers. Based on symmetry of electrodes, superapacitors can be classified as symmetrical and assymetrical type. Mechanism of energy storage occurs for EDLC based supercapacitor is due to charge separation at electrode/electrolyte interface or electrostatic adsorption-desorption of ions on electrode high surface area from electrolytes while in case of pseucapacitors, fast and reversible redox reaction of electroactive specimens on both electrode surface (electroactive material) and bulk phase results in high capacitance and high energy density. Supercapacitors have been noted to deliver attractive features in the range of high power delivery capability (>10 kW kg-1), long cycle life (>100 000 cycles), fast charge-discharge kinetics (few seconds) thus providing the bridge between traditional dielectric capacitors and batteries. For an electrochemical double layer supercapacitor charge accumulation in the electric double-layer can be expressed in as: C = Q/V = ε0εrA/d where Q is the total charge on the plates, V is the voltage imposed across the capacitor, A is the specific area of the electrode/electro interface, ε0 = 8.854 ×10-12F m-1 is the permittivity of free space and εr is the medium dielectric constant and d is the effective thickness of the electrical double layer. For pseudocapacitors, the pseudo-capacitance of metal oxides can be calculated from the CV data according to the following
equation: C = (∫ idV ) / v(ΔV ) where i is the response current, ν is the potential scan rate (v/s) and ΔV is the applied potential.3 Material properties like high conductivity, low resistivity, large surface area, chemical stability are meant for such applications. First supercapacitor was RuO2 but problem of scarcity, toxicity hindered its popularity as promising material for applications. Several oxides, transitional metal oxides are explored for such purpose. Complex oxide of NiCo2O4 is found to be most promising alternative supercapacitors due to its higher electrical conductivity and electrochemical activity than single oxides. But low capacitance, low intrinsic electrical conductivity actually hinders Ni-Co-O spinel based supercapacitors. Thus, optimum cyclic stability, high capacitance, excellent rate capability is a challenge for Spinel Ni-Co-O based supercapacitors. It is observed that CNT among all carbonaceous material posses some characteristics for its suitability for supercapacitor applications.1,2
Electrochemical detection of insulin is of utmost importance in present research time for the impact of this protein molecule in various human physiological activities. Moreover, electrochemical detection reduces analysis time and provides high sensitivity. Mono-oxides have been replaced by RuOx, InOx for acting as electrocatalyst to study oxidation of insulin. Mixed oxides due to their synergetic effect have tendency to improve such properties and amongst them transitional metal Ni-Co-Oxides is noted very efficient in present of alkaline electrolytes. Addition of carbon based material is noted to have improved electrocatalytic behaviour due to specific surface area and porosity. Composite of Ni-Co-O powder with CNT is added into the pores of Nafion membrane while ameperometry, Cyclic voltammetry electroanalytical studies are carried to note the behaviour of insulin. It is noted that electrocatalytic activity for insulin detection is improved with CNT addition on Ni-Co-Oxides. Presence of entangled network and porous structure of CNT causes enhanced electrocatalytic activity due to higher electrochemical reversibility of the oxide-nanocomposite material than simply metal oxide material. Entangled network and porosity aids in well accessible electrode/electrolyte interface with accelerated charge transfer process.8 Metal sulphides having novel nanostructures of cubic Cobalt Nickel sulphides, hexagonal nickel sulphides (Ni17S18) synthesized by one step hydrothermal method is also noted to possess electrochemical properties to exhibit energy storage and conversion.10 It is also noted that different carbon based materials like CNT, Graphite nanoparticles, graphene electrodes are also prominent material for paper supercapacitors. Paper supercapacitors based on CNT electrodes exhibit higher capacitance value of about 411F/g compared to graphite nanoparticles and graphene.11 In the present article, a brief glimpse is focussed upon CNT-Ni-Co-O based composite material for their advantages over single phase CNT, Ni-Co-O as energy material in terms of supercapacitor applications.
Experimental Synthesis Procedures
Nickel cobalt oxide/CNT hybrid as high performance electrocatalyst for metal/air battery was reported by Hui Zhang et al. CNT were functionalised by acid to generate surface functional group followed by calcination, purification by acid. Composite was prepared by facile-hydrothermal route followed by appropriate thermal treatment. Nitrate salts of Co, Ni and hexamethylenetetramine were used as precursors for spinel, while CNT undergoes ultrasonication in ethanol for proper dispersion and attachment to generate the composite.4 The Nickel Cobalt Oxide Single walled CNT nanocomposite was also noted for superior cycling stability and super capacitor applications by Xu wang et.al. The precursors of Co-Nitrate, Ni-chloride, urea, ethanol were used for the synthesis. Water/ethanol ratios used were 4:1, 1:1, 1:4, along with mass variation of SWCNT. Resultant solution prepared were made to undergo hydrothermal reaction in autoclave at 80ºC for 14 hours, followed by drying in centrifuge collected precipitate at 60ºC and sintered finally at 400ºC for 3 hours.5
Chao Wei et al., utilizes hydrothermal process followed by calcinations to prepare NiCo2O4/NiO electrode material for electrochemical storage applications.6 High purity of Ni(Ac)2, Co(Ac)2 of 1:2 ratio along with Urea were added to solution of 35 ml mixture of isopropanol, triethylene glycol. On stirring for 1 hour solution turns pink followed by addition of hexamethylene tetra amine into the mixed solution and then further stirred for 30 minutes. Resultant solution was then transferred to teflon coated autoclave for reaction at about 160°C for 12 hours followed by annealing at 450°C for 2.5 hours.6 Xiaocheng Li et. al have tried to synthesize hierarchial three dimension self supported NiCo2O4/CNT core shell networks as electrodes for high performance supercapacitors. Initially CNT was made to grow on Nickel foam acting as current collector for supercapacitor electrode. Nickel foams were subjected to cleaning by 2% dilute HCl, acetone, deionized water within an ultrasonicator bath followed by drying with Nitrogen gas. CNT were made to grow by flow of C2H2 under hydrogen atmosphere in CVD setup. After formation of CNT on Nickel foam, air plasma treatment was carried to obtain hydrophilic surface for electrodeposition of NiCo double hydroxides. Electrodeposition was carried with standard 3 cell electrode configuration at a cathodic potential of -0.7V, using saturated calomel electrode as reference electrode, platinum plate as counter electrode and CNT/NF as working electrode using aqueous solution of Ni-Co, Na nitrate as electrolytes. After electrodeposition calcinations was carried at 300°C for 2 hours with ramp rate of 1°C/min to form NiCo2O4.1 Qianqian Li et al. have focused on Ni-Co-Oxide coated CNT as additives of activated Carbon electrode for supercapacitors. CNT was synthesized utilizing CVD process and pretreated to develop Ni-Co nanocomposites. Initially boiling in nitric acid was carried for 3 hours to undergo purification and surface modifications. For sensitization, pretreated CNTs were first put into SnCl2 solution, stirred at room temperature, followed by activation by putting it into PdCl2 solution. Activated CNTs were then collected by means of filtration and then made to undergo electroless plating of Ni-Co at temperature of 90°C, ph value 9.0 for 20 minutes duration in solution of Ni-chloride, sodium hypophosphite and others.7 It was observed that CNT-Nickel Cobalt modified oxide can be utilized for electrocatalytic oxidation for determining insulin as observed by Adina Arvante et al., It was modified by attaching Nafion to the nanocomposite. Nafion bonded electrodes were prepared by mixing correct amount of Nafion to NiCoO2 catalyst inside a small bottle undergoing dispersion, stirring with ultrasonication. Microvolume 2μl of resulting mixture was dropped into screen printed electrode while the composite electrode was obtained by addition of CNT of concentration 8mg/mL to NiCO2/Niafon solution.8
Electrochemical Properties by Cyclic Voltametry and Characterization
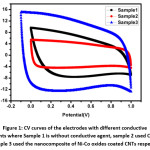
Figure 1: CV curves of the electrodes with different conductive agents where Sample 1 is without conductive agent, sample 2 used CNTs and sample 3 used the nanocomposite of Ni-Co oxides coated CNTs respectively.7
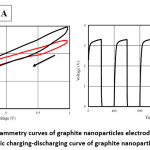
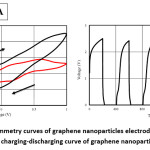
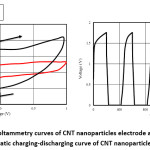
High performance supercapacitor electrodes were fabricated by preparing CNT-Ni-Co-O coated CNT as additive of Activated Carbon electrodes. The above was noted by Qianqiang Li et al., where the sample was prepared by electroless coating of Ni-Co oxide over CNT followed by characterization of TEM to determine the dispersion of CNT, XRD for phase analysis and Cyclic voltametry, ac impedance analysis and charging-discharging for electrochemical properties. CV test was carried at sweep rate of 10mV/s without observation of any redox reaction peaks indicating double layer capacitance behavior for all samples. For Ni-Co oxide Coated CNT, low pseudo capacitance was noted during discharge process. The integrated area under CV was largest for this case indicating higher specific capacitance caused due to structural modification and higher interface densities. Ni-Co coated CNT activated carbon exhibits highest conductivity in compare to CNT coated sample and one without CNT. Formation of CNT structure network in the electrode results in reduced resistivity and moreover the addition of nanocomposite changes the interface between the activated carbon and additives.7 Paper supercapacitor was prepared using push coating method involving barium titanate, N-Methyl-2-pyrolidone mixture on paper substrate. PVA was used as both separator and electrolyte for the purpose while the gel electrolyte film was prepared by mixing PVA and H3PO4. Push coating method ensures ensures formation of carbon layered material on the surface of the PVA film layer. CNT was added by dispersion after surface activation by sulphuric acid and nitric acid. With the potential range -1V to +1V having voltage scan rates of 20mV/s and 150mV/s cyclic voltammetry was carried to obtain the specific capacitance. It was noted that current increases from -1.1 A to 2.17 A for scan rate of 150mV/s while the current increases from -0.3 A to 0.62 A for scan rate of 20mV/s. Specific capacitance was obtained using the formula Cm = [mS(V2 – V1)-1 ∫V1V2 IdV where S is the voltage scan rate and m is the mass of the electrode material. Using galvanostatic method specific capacitance was calculated using the formula Cm = I[m(dV/dt)]-1 where dv/dt represents absolute slope value from discharging curve. Using the first formula specific capacitance of graphite nanoparticles were noted to be 79F/g, 380F/g for scan rates of 150mV/s and 20mV/s respectively. It was also noted from charging-discharging behaviour of galvanostats at current density of 0.06mA/cm2 graphite nanoparticles act like conventionalcapacitors while specific capacitance was noted to be about 163F/g. It was noted for grapheme nanoparticle electrodes that specific capacitance from CV curves at scan rates of 20mV/s and 150mV/s to be about 410F/g and 87F/g respectively. Similarly, specific capacitance from Galvanostatic charge-discharge was noted to be about 310F/g within the range of CV curve. The specific capacitance for CNT electrodes was noted from CV and galvanostaic charging-discharging principles. From CV analysis, at about scan of 150mV/s a nearly rectangular profile was noted. Specific capacitance value was about 164F/g, 411F/g for scan rates of 150mV/s and 20mV/s respectively. Specific capacitance of paper capacitor was noted to be about 520F/g from galvanostatic charging discharging and the value was higher in compare to graphite, graphene nanoparticles. The energy density, power density calculated was noted to be 79 Wh/Kg and 11KW/Kg for graphite nanoparticles, 81Wh/kg, 12Kw/Kg for grapheme nanoparticles while for CNT nanoparticle electrodes it was 91Wh/kg and 13.6Kw/Kg respectively.11
Figure 2: A) Cyclic voltammetry curves of graphite nanoparticles electrode at 20mV/s, 150m V/s B) Galvanostatic charging-discharging curve of graphite nanoparticle electrodes11
Figure 3: A) Cyclic voltammetry curves of graphene nanoparticles electrode at 20mV/s, 150m V/s B) Galvanostatic charging-discharging curve of graphene nanoparticle electrodes11
Figure 4: A) Cyclic voltammetry curves of CNT nanoparticles electrode at 20mV/s, 150m V/s B) Galvanostatic charging-discharging curve of CNT nanoparticle electrodes11
Xiaocheng Li et al., noted high performance supercapacitor electrodes by creating 3-D hierarchial Self supported NiCo2O4/CNT core shell networks. 3-D hierarchial NiCo2O4/carbon nanotubes/Nickel foam had been developed by electrodepositon of Ni-Co layered double hydroxides on CNT surface. Coreshell structure formed was evident from FESEM studies (fig5).
Figure 5: FESEM image of CNTs development in hairy form over Nickel Foam substrate1
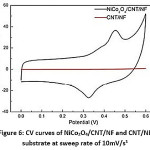
CV curve of NiCo2O4/Nickel Foam electrode at various sweep rates were obtained and it was characterized by a pair of defined redox reaction peaks. CV curves of NiCo2O4/CNT/NF electrode exhibit redox reaction peaks between 0.2-0.6V, while typical rectangular shape region was noted within potential range 0-0.2V which was linked to the electrical double layer between NiCo2O4 and hydroxyl ions. It was noted both Electric Double Layer Capacitors and Faradic reactions were involved during the energy conversion processes of NiCo2O4/CNT/NF electrodes. Comparing CV curves of NiCo2O4/CNT/NF and CNT/NF substrate contribution of EDLCs from CNTs could be evaluated at sweep rate of 10mV/s.1
Figure 6: CV curves of NiCo2O4/CNT/NF and CNT/NF substrate at sweep rate of 10mV/s1
CV curve of CNT/NF substrate was nearly horizontal line along the potential axis indicating a low EDLC value. Reversible adsorption of electrolyte ions on surface of mesoporous NiCo2O4 rather than from CNT/NF substrate causes remarkable EDLC. CNT provides a highly conductive skeleton for depositing mesoporous NiCo2O4 and form the composite core-shell structure. Peak potential of the composite shifted only by 50mV even with five time increase in sweeping rate. It concludes a superfast electronic transport rate and good rate capability of the electrode material.1
Chao Long et al synthesized amorphous Ni-Co binary oxide hierarchial structure by chemical deposition and calcinations without any involvement of template and complicated precursors. CBD process generates nanomaterials after calcinations with porous structure due to removal of gases after breakdown of the precursors. Due to porous structure high specific area is present while large volume of Ni-Co oxides generated causes rapid penetration of electrolyte and sufficient electroactive sites for redox reactions. Amorphous material exhibit good electrochemical properties due to their excellent rate capability and high cyclic stability though in general amorphous were not stable for electrochemical capacitor applications. Amorphous material sometimes exhibit comparable specific capacitance leading to fairly large faradaic storage of pseudocapacitors with no phase transitions. Larger channels and more reaction sites were noted for amorphous material due to random fluctuations in atomic positions for amorphous material. This cause improvement in ion diffusion and effective intercalation/deintercalation processes. The electrochemical properties of the as synthesized sample were carried in 3 electrode mode using 6M KOH aqueous solution as the electrolyte. The cyclic voltmeter test and galvanostatic charge-discharge test was carried between 0 and 5V at 25°C. From the CV curve studies, researchers obtained specific capacitance of Ni-Co oxide to be about 736F/g while for Ni-O and Co-O exhibits 243 and 225F/g respectively. At same scan rate of 10mV/s specific capacitance was noted for 5 samples to be about 1101, 396, 736, 838 and 606F/g respectively. The specific capacitances were calculated at different scan rates of 5, 10, 25, 50 and 10mV/s. Only one sample designated as S4 (higher Ni content lower Co) exhibits better electrochemical performance. NiO plays a significant role on the electrochemical performance of the sample. Galvanostatic charge discharge test for Ni-Co oxides along with Ni-O, Co-O are studied at same current density. The results obtained are in conformity with CV studies. Electrochemical impedance Spectroscopy results also indicate electrochemical where all EIS curves are composed of depressed circle in high frequency region while at low frequency a straight line is obtained. Vertical nature curves at low frequency indicate pure capacitance performance electrochemical capacitors where a cyclic life measurement on metal oxide (hydroxide) indicates high stability due to agglomeration of particle. For evaluating performance of supercapacitors energy density and power density are two vital parameters to be enhanced diffusion and adsorption of electrolyte ions. Massive structural defects on the surface of binary oxide lead to more and fast faradic process while novel amorphous structure leads to enhanced electrochemical efficiency due to introduction of more transportation channels.9 Yangwen Liu et al. reported that capacitive performance of EDLC supercapacitors were guided by pore volume, surface area of electrode materials which laid stress upon porous carbon based materials. These material posses specific nanoarchitecture having certain characteristics like large surface area, chemical stability, availability of active sites, high electron conductivity to render it fit as electrode material. Several carbon based materials like carbon spheres, carbon nanofibers, activated carbon, carbon nanotubes, carbon nanoparticles were regarded as possible material for optimizing EDLC performance. Porous hollow carbon nanofibers were prepared initially after pyrolysis of electrospinning of PVP/PSN precursor fibres followed by activation with NaOH and activation by carbonization treatment. Electrochemical behavior was analysed by CV, glavanostatic charge/discharge, Electrochemical impedance spectroscopy measurements. SEM image executes the morphology of the synthesized samples. It was noted that carbon nanofibers have fascinating hollow formation having diameters of hollow fibers to be about 0.4 to 1.4μm. Cross-sectional view of the nanofiber exhibits tubular structure. It was noted that sufficient porous structure were present on the wall revealing hollow and porous duple morphology of the nanofibers. The further detailed microstructure was investigated by TEM and HRTEM analysis. The hollow structure along with porosity on the wall was justified by intensive contrast between light centre and dark edges. Moreover closer insight into hollow fiber structure reveals irregular arrangement of pore structure on the wall causing hierarchial structure of the material. CV curve of the samples exhibit nearly a rectangular shape at scan rate of 5mV/s thus displaying electric double layer capacitance behavior. With rise in carbonization temperature, area under CV curve increases indicating higher capacitance. Moreover, CV curve of higher carbonized samples at higher scan rates of 200mV/s executes quasi rectangular shape. GCD curves of samples triangular symmetrical shapes while higher carbonized temperature displayed higher capacitance than others at specific current density of 1A/g. The capacitive behavior was studied with two-electrode system in the potential range of 0- 1.2V at different electrolytes. CV curves exhibit analogous rectangular shape with increase in scan rate. At higher scan rate of 1600 mV/s good rectangular shape was maintained for the CV curve without any redox peaks.12
Conclusion
CNT-Ni-Co-O based composite is found to be prepared by facile hydrothermal, hydrothermal, electrodeposition, chemical bath deposition electrocatalytic oxidation process. All the process is found to be effective depending on the morphology and category of sample preparation. Cyclic voltammetry curves and galvanostatic charge discharge processes are noted to be very efficient method for determining electrochemical performance of CNT Ni-Co-O based composites. Porous and hierarchial morphological features are found to increase electrochemical performance. Detailed morphology analysis by TEM, HRTEM is found to be effective to understand the nature of porosity or hollowness which is useful for supercapacitance behaviour. Among carbon based material used for electric double layer capacitance, CNT is found to be most effective due to its high surface area, chemical stability, enhanced conductivity.
Acknowledgement
The author is likely to acknowledgement Kazi Nazrul University Asansol for providing technical support to prepare the manuscript.
Funding Source
There is no funding source for the above work.
Conflict of Interest
There is no conflict of interest.
References
- Xiaocheng Li, Wei Sun, Liqun Wang, Yongdong Qi, Tieming Guo, Xinhong Zhao and Xingbin Yan, Three-dimensional hierarchical self-supported NiCo2O4/carbon nanotube core–shell networks as high performance supercapacitor electrodes, RSC Advances. 5, 11, 7976-7985 (2015)
- Farzaneh Hekmat, Saeed Shahrokhiana and Sajad Rahimi, 3D flower-like binary nickel cobalt oxide decorated coiled carbon nanotubes directly grown on nickel nanocones and binder-free hydrothermal carbons for advanced asymmetric supercapacitors, Nanoscale. 11, 6 2901-2915 (2019)
- Di Chen, Qiufan Wang, Rongming Wang and Guozhen Shen, Ternary oxide nanostructured materials for supercapacitors: a review, J. MATER. CHEM. A. 3, 19 10158-1173 (2015)
- Hui Zhang, Hang Qiao, Haiyan Wang, Nan Zhou, Jiajie Chen, Yougen Tang, Jingsha Li and Chenghuan Huang, Nickel cobalt oxide/carbon nanotubes hybrid as a high-performance electrocatalyst for metal/air battery, Nanoscale. 6, 17, 10235-10242 (2014)
- Xu Wang, Xuanding Han, Mengfang Lim, Nandan Singh, Chee Lip Gan, Ma Jan and Pooi See Lee, Nickel Cobalt Oxide-Single Wall Carbon Nanotube Composite Material for Superior Cycling Stability and High-Performance Supercapacitor Application, J. PHYS. CHEM. C. 116, 23, 12448-12454 (2012)
- Chao Wei, Ying Huang, Menghua Chen, Jing Yan, Wen Yao and Xuefang Chen, Fabrication of porous nanosheets assembled from NiCo2O4/NiO electrode for electrochemical energy storage application, J. COLLOID. INTERF. SCI. 504, 15, 1-11 (2017)
- Qianqian Li, Jipeng Chen and Li Zhang, Proceedings of the 13th IEEE International Conference on Nanotechnology, Beijing, China, August 5-8, 2013
- Adina Arvinte, A. Caroline Westermann, Adama Marie Sesa and Vesa Virtanen, Electrocatalytic oxidation and determination of insulin at CNT-nickel–cobalt oxide modified electrode, SENSOR ACTUAT. B-CHEM. 150, 2, 756-763 (2010)
- Chao Long, Mingtao Zheng, Yong Xiao, Bingfu Lei, Hanwu Dong, Haoran Zhang, Hang Hu and Yingliang Liu, Amorphous Ni–Co Binary Oxide with Hierarchical Porous Structure for Electrochemical Capacitors, ACS. APPL. MATER. INTER. 7, 44, 24419-24429 (2015)
- C Karthikeyan, R Dhilip Kumar, J Anandha Raj and S Karupuchamy, One pot and large-scale synthesis of nanostructured metal sulfides: Synergistic effect on supercapacitor performance, Energ Environ. https://doi.org/10.1177%2F0958305X19899373, 1-18, (2020)
- L. Fekri Aval, M Ghoranneviss and G Behzadi Pour, High-performance supercapacitors based on the carbon nanotubes, grapheme and graphite nanoparticles electrodes. Heliyon. 4, 11, Article e00862 (2018)
- Yangwen Liu, Qiao Liu, Lin Wang, Xianfeng Yang, Weiyou Yang, Jinju Zheng and Huilin Hou, Advanced supercapacitors based on porous-hollow carbon nanofiber electrodes with high specific capacitance and large energy density. ACS. APPL. MATER. INTER. 12, 4, 4777-4786 (2020)
Views: 1,017
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal