Effect of Humidity on Electrical Conductivity of Graphite Nanocomposite Based Electrodes: A Review
Sameena Mahtab1 , Pragati Joshi1, Bhagwati Arya1, M.G.H. Zaidi1*
, Pragati Joshi1, Bhagwati Arya1, M.G.H. Zaidi1* and Tanveer Irshad Siddiqui2
and Tanveer Irshad Siddiqui2
1Department of Chemistry, G.B. Pant University of Agriculture & Technology, Pantnagar India-263145
2Department of Chemistry, D.I.T University, Mussoorie Diversion Road, Makkawala, Dehradun, Uttarakhand
Corresponding Author Email: mghzaidi@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170103
Article Publishing History
Article Received on : 29 March 2020
Article Accepted on : 30 April 2020
Article Published : 30 Apr 2020
Plagiarism Check: Yes
Reviewed by: Narendra Kumar Ahirwar
Second Review by: Karthick S
Final Approval by: Bhaskar Bhattacharya
Article Metrics
ABSTRACT:
We have reviewed recent progress on various types of humidity sensors as it is one of the most significant issues in various areas of sensing appliances such as instrumentation, charge storage automated systems, industries and agriculture. Various effective approaches have been discussed to develop ceramic, semiconducting and polymer based graphite sensors. It was found that graphite based nanocomposite materials have unique potential for detecting humidity due to specific structure, high electrothermal conductivities, good mechanical properties, low cost and ultrahigh surface area that increases applications in the field of energy storage devices.
Graphical Abstract
KEYWORDS:
Humidity; Graphite; Electrochemical Capacitor; Nanocomposites; Electrodes
Copy the following to cite this article:
Effect of Humidity on Electrical Conductivity of Graphite Nanocomposite Based Electrodes: A Review. Mat. Sci. Res. India; 17(1).
|
Copy the following to cite this URL:
Effect of Humidity on Electrical Conductivity of Graphite Nanocomposite Based Electrodes: A Review. Mat. Sci. Res. India; 17(1). Available from: https://bit.ly/2SlOroU
|
Introduction
Electrodes are the integral part of devices related to power production, communication, charge storage and light harvesting. They play an important role in powering systems of portable electronic devices like batteries and supercapacitors.1 They have attracted worldwide attention due to various functions as energy storage appliances, in digital communication devices, digital cameras, mobile phones, electric tools and solar cell.2-6 They can be categorized as pseudo capacitor or electric double layer capacitor (EDLC). EDLCs focuses on charge storage across double layer of the electrode in carbon based electrode.7 Pseudo capacitance involves faradic processes it stores energy like EDLC but also performs reaction between electrode materials and ions in restricted potential window such as oxides of nickel, manganese, vanadium.8
The performance and life of electrical and electronic devices is best dictated of the stability of electrode working under hazardous conditions. Functionality and performance are compromised during their working in humid environment and heat production due to long term application. Therefore due to numerous applications of humidity sensors in agriculture, medical fields, semiconductor industry moisture levels are constantly examined. With emerging technologies low power consumption based miniaturized humidity sensors can be designed for various types of electrochemical studies.9 These can be categorized as organic/inorganic based sensors, ceramic type (Al2O3, SiO2), semiconductors,(TiO2, SnO2, ZnO), organic polymer hybrid sensors and pervoskite sensors.10 Present century has witnessed significant advancements in the development of power producing and power storage accessories for better performance of electronic devices applicable in communication. For such reason, there has been a continuous need of electrode that may impart enhancement and longevity of devices for best possible application in communication, energy storage and light harvesting.11-13
However, limited exploration has been made on impact of humidity on electrical conductivity of graphite based electrodes.14-16 Due to unique properties and extraordinary atomic arrangement of graphite it has wide applications in energy storage, chemical sensors, optoelectronics and molecular separation. This review focuses on recent advances in humidity sensors and effect of humidity on electrical conductivity of working electrodes.
Fabrication of Electrodes
Electrodes are chemically designed through deposition of a mixture of graphitic materials with electrically conducting inorganic or organic additives in presence of binders over a conducting surface.
Components of surface coating materials
Graphitic materials
Materials employed for developing graphitic electrodes are various forms of natural, expanded graphite, carbon black, graphene fullerenes and tubular nanostructures.17-19 Graphite is naturally most abundant allotrope of carbon with two dimensional (2-D) hexagonal lattices in which sp2-bounded carbon is present. Flat sheet like structure of graphite bound to each other by weak Vander Waal forces, in which the electrons are delocalized, due to thin gap between valence and conduction band it is a good conductor of electricity. 20 Graphite has wide applications in the development of batteries,21-22 sensor 23-25 supercapacitors,26-28 and nanocomposites.29
Structure of graphite consists of hexagonal rings forming thin parallel plates (graphenes) in which each carbon atom is covalently bonded to three other atoms in the plate (the angle between two bonds is 120°). The outermost electron shell of a carbon atom has four valence electrons, three of which are used by the covalent bonds. The forth valence electron does not take part in covalent bond formation and may be easily displaced from the electron shell making graphite electrically conducting.
Polymers with enhanced solubility in polar solvents are employed in trace proportions as binders for graphitic materials to achieve the stable conducting surface over metallic substrates.30 In recent years use of graphitic materials as conducting matrix has numerous applications including ceramics, semiconductors, solar cells, light emitting diodes.
Polyindole
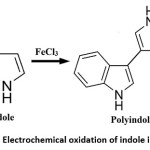
Composite polymers such as polyindole, polypyrolle, polyacrylonitrile etc are also employed as conducting filler for graphitic electrodes to enhance their I-V response under varying voltages.31-36 In early 1976 initial work on chemical polymerization of PIN from indole can be traced. Tourillon et al in 1982 firstly reported the formation of PIN by electrochemical formation. PIN is a conducting electroactive polymer, which can be obtained by electrochemical oxidation of Indole in a variety of electrolytes and chemical polymerization through oxidants.37-39 PIN have N-containing five member aromatic compound which is responsible for its easy synthesis. PIN process high redox activity, low decomposition rate and batter air durability but poor mechanical properties (Fig. 1).40
Figure 1: Electrochemical oxidation of indole into PIN
In recent times, the application of PIN as an electroactive material has been realized for the development of respective NCs in combination with carbon NCs electrodes for communication, energy storage and light harvesting complexes.41 PIN-based rechargeable batteries have been reported for fast charging and discharging due to high electromotive force together. It is believed that PIN uses protons as charge carriers to speed up the electrical conductivity.42
Tungsten carbide
The conductivity of polymers may be improved greatly by modifying them with electrically conducting fillers. Such fillers may be metals or their molecular derivatives with chacogens and alike molecules.43-44 Recently, metal carbides have received increasing attention as electrically conducting fillers for polymers to derive the NCs.45-46 Such NCs are blended with graphitic materials such as coke, graphene, graphite oxide and carbon nanotubes to develop the electrodes for energy storage devices and production of energy. 47-48
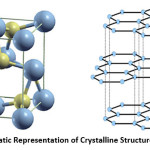
Figure 2: Schematic Representation of Crystalline Structure of (a) WC (b) G
Fig. 2 (a) and (b) represent crystalline structure of WC and graphite respectively. WC exhibits a hexagonal structure made of a grid of tungsten and carbon. It’s most striking properties are a high density and a high melting point of 2600 °C, a high hardness as well as the metal-like, high values of electric and thermal conductivity. The WC grid causes a certain plasticity and high breaking ductility while maintaining the high hardness of tungsten carbide. Exposed to air, WC corrodes only at temperatures above 600 °C. Furthermore, besides its high hardness at ambient temperature and at temperatures above 1000°C, the ability to bind metals such as cobalt, nickel and iron is responsible for its economical importance. WC is mainly used for the production of hard metals. 49-51
Effect of humidity on nanocomposites based electrodes
Humidity is of great interest to researchers due to their important role in both basic and applied research.52 This paper reviews reported studies on different humidity sensor fabrication technologies, principles of materials and applications (graphical abstract). The practical performance of electrode such as conductivity, sensitivity, stability and reliability depends on structure of polymer. The performances of electronic devices are affected in humid environment. Table 1 shows summary of the sensing performance of humidity sensors based on various working electrodes.
Perovskite type humidity sensors
Study of the humidity sensing behaviour of porous perovskite films and bulk materials continues to give rise to novel results. Quantitative and systematic investigation of perovskite degradation has been conducted. It was found that rapid decomposition of CH3NH3PbI3 semiconductor in moist air limits their commercial utility as an electrode material for Perovskite solar cells. The phase changes in Perovskite degradation under humid environment was monitored through in situ absorption spectroscopy and XRD. This has revealed the formation of [PbI6]4- octahedral as a first degradation product under humid environment. In subsequent studies, it was found that the lead role of moisture on formation of discrete hydrate phases that implies to corrosion of electrodes11,15 Effect of humidity and temperature on stability of encapsulated planar-structured CH3NH3PbI3 perovskite solar cells has been investigated. The device has shown significant degradation under prolonged exposure temperature up to 85°C and RH up to 80%. The degradation of CH3NH3PbI3 was revealed through SEM and XRD53.
Nanocomposites based humidity sensors
Nano composites with improved optical and electrical properties through insitu polymerization of GNP/silicone composites has been synthesized. Microstructure of composite was investigated through XRD, FESEM, TEM and PL spectra. PL spectra reveal maxima at blue wavelength with enhanced rate of electron-hole recombination.19 Electrically and thermally conductive TEM in combination with XRD reveals a decrease in thickness of G nanoplatelets from 60 to 35 nm under mechanical mixing. NCs derived through dispersion of 20 wt% G nanoplatelets shows DC conductivity of 0.360 S.m-1 that was higher over Si (8.33×10-13 S.m-1) whereas became insulating at 15 wt%.54
Polymer nanocomposites based humidity sensors
Electrically conducting NCs of poly (3-methoxythiophene) and nickel oxide through chemical oxidative polymerization has been synthesized. Conductivity of the composites was found to be the function of concentration of NiO and was percolated up to 4.2× 10−7 Ω/cm−1.Diversified spectral and microscopic method reveals clustered morphology of NCs with dispersion of crystalline nanocrystalline (5 nm) NiO into polythiophene matrix.55 Mo and WS2 particles over PANI for electrorheological application has been coated. Coating of WS2 over PANI was revealed through FTIR and Raman spectra. Conductivity of WS2 was increased from 0.056 Scm−1 to 0.98 Scm−1 on coating with PANI. However, this was decreased to 6.3 × 10−6 Scm−1 with PANI base. TGA reveals improved thermal stability of films.44
Humidity sensors containing polyindole
Over stainless steel fabric, binder free electrodes have been fabricated through electrospinning of PIN/CNT nanofibers. Microanalysis reveals restricted surface area and inactive morphology of electrodes. Electrodes display moderate DC conductivity with improved charge storage. DC conductivity of electrodes was improved through addition of 10 wt% of CNT.A single step supercapacitor system was developed with 95% capacitive retention till 2000 cycles. Under cyclic conditions the energy density and power density of electrode were 17.14 W h Kg-1 at 426 W Kg-1 respectively.56 NCs with improved spintronic behavior, thermal stability and Tg has been synthesized through insitu polymerization of PIN in presence of PVA and magnetite for electrical and dielectric applications. Effect of magnetite concentration on conducting behavior of composite was investigated. This has revealed the highest conductivity of NCs at 10 wt% of magnetite. With frequency, NCs reveals a characteristic decrease in dielectric constant and loss tangent that was enhanced with concentration of magnetite. Microstructure of NCs was investigated through SEM and XRD.57 NCs through insitu polymerization of indole has been prepared in presence of chloro-auric acid
Polymerization was conducted under surfactant free micro emulsion system under electrically conductive control of AFM. Microanalysis reveals core shell morphology of NCs highly populated, size controlled, stable mono dispersed Au nanoparticle in core of PIN.37 PIN/CNTs composite with The battery achieves about 80–70 mAh/g at discharge current densities of 10–103 A/m2. Surface measurements of composite were done via BET, XRD, XPS, SEM, TEM, FTIR and Raman spectroscopy.58 Li-ion battery has been fabricated using Na foil, electrospun poly (5-cyanoindole) nanofiber and NaPF6 as anode, cathode and electrolyte solutions respectively. Fabricated battery was characterized through Cyclic voltammetry, electrochemical impedance spectroscopy shows electromotive force of 3.4V, discharge capacity of 106–75 mAh/g.59 Li-ion battery has been synthesized with cyclic life of 103A/m2 and discharge depth 60% at 25oC with 30,000 time’s cyclic stability using PIN cathode, Li anode and LiBF4 with electromotive force of 3.0V as electrolyte medium. Battery renders the discharge @10-103 A/m2 with power storage of 80-70mAh/g. Bhagat and Dhokane has prepared polyvinyl acetate (PVAc) through insitu polymerization of indole in presence of ferric chloride. This has afforded PVAc films with DC conductivity of 4.46×10-6 S/cm at 383K under two point probes. The formation, composition and morphology of films were well investigated through XRD, FTIR, UV-Vis spectra, FE-SEM, EDAX.60 In subsequent years, the same class of composites with 30 wt % of PIN has been re-synthesized. Composites were investigated for AC conductivity. It was found that the AC conductivity of composites was 1.89×10-6 S/cm at 1MHz.TG-DTA reveals enhanced thermal stability of composite with endo and exo DTG peaks at 11.844-25.775KJ/g mol and 54.454-61.603 KJ g mol. FESEM reveals cauliflower like morphology of composites with soft circles.61
Humidity sensors based on metal oxides
Boron/ZnO composite film was developed through chemical vapor deposition method. Nano composites were investigated for stability under damped heat treatment. Electrical and optical characterization of films reveals increase in the scattering of electron gain boundary. Films show an increase in resistivity during exposure of humidity due to decrease in Hall mobility.9 The stability of electrodes of Li-ion batteries has been investigated. The humidity experiments were conducted over LiFeSO4F as cathode material. Complete degradation of cathode material in FeSO4.nH2O (n=1, 4, 7) and LiF in environments with relative humidity greater than 62%. A rapid decomposition of LiFeSO4F into FeSO4·nH2O (n = 1, 4, 7) and LiF was observed in highly humid environment (>62% RH at 25˚C) till destruction of electrodes.10 Degradation of organic photovoltaics under humid environment has been done. Experiments performed shows drop performance of photovoltaic below 40% within only 45 min under o RH of 85%.62 Heteropolyacid derived membrane electrode assembly (MEA) for operation at 120oC and 35% relative humidity has been developed. Assembly material was characterized through spectra, CV, EIS and TGA and was found stable under humid environment.
Humidity Sensors Applications
Effect of Humidity is essential for various applications at industrial, environmental, domestic, medical, and agriculture sites. Conductivity of electrodes under accelerated heat and humid environments is hereby compromised, that alters the performance of storage and disbursement of charge from batteries and capacitors. Most of the workers work on development of humidity sensors63-65 but limited reports are available on impact of humidity over stability and conducting behavior of semiconducting electrodes.66-68
Table 1: Summary of the sensing performance of humidity sensors based on various working electrodes
|
S. No.
|
Coating Material
|
Preparation Method
|
Conductivity
|
Operating Temperature
|
Characterization
|
Response
|
Ref
|
|
1.
|
CH3NH3PbI3
|
Electrodeposition
|
_
|
85o C
|
XRD, AAS, SEM
|
80%
|
[53]
|
|
2.
|
MMA/ZnS
|
_
|
0.360 Sm-1
|
R.T
|
SEM, TEM, XRD
|
_
|
[54]
|
|
3.
|
Thiophene/ Nickel oxide
|
Chemical oxidative Polymerisation
|
4.2×10-7 Scm-1
|
R.T
|
SEM, FTIR, XRD
|
_
|
[55]
|
|
4.
|
Mo/ WS2/PANI
|
Electrodeposition
|
6.3 × 10-6 Scm-1
|
30o C
|
FTIR, Raman
|
_
|
[44]
|
|
5.
|
PIN/ CNT
|
Electrospining
|
_
|
28o C
|
SEM, XRD, FTIR, Raman
|
95%
|
[56]
|
|
6.
|
PIN
|
Electrodeposition
|
1.89 ×10-6Scm-1
|
R.T
|
FESEM, XRD
|
80%
|
[61]
|
|
7.
|
Graphene/ Methyl red
|
Electrohydrodynamic
|
_
|
R.T
|
_
|
5- 95%
|
[69]
|
|
8.
|
Graphene/ TiO2
|
Sol- gel
|
_
|
25o C
|
XRD, FTIR, SEM
|
12- 90%
|
[70]
|
|
9.
|
GO/MWCNT
|
Drop Casting
|
_
|
25o C
|
SEM, Raman
|
11- 97%
|
[71]
|
|
10.
|
RGO/ Cellulose
|
Pour Casting
|
_
|
25- 45o C
|
XRD, SEM, FTIR
|
25- 90%
|
[72]
|
Conclusions
This review summarizes numerous humidity sensors based on metal oxides, pervoskite, ceramic, polymer metal nano composite and semiconducting materials. It also provides a simplified method of fabrication of NCs derived WEs with enhanced thermal stability, electrical conductivity and application under humid environment. Graphene based polymer nanocomposites develop excellent humidity sensors having high sensitivity, fast response and long term stability The electrodes may find their potential applications for energy production and storage devices.
Acknowledgements
Authors are highly thankful to GB Pant University of Agriculture & Technology for providing space and financial support to complete this research work.
Funding
The work is financially supported by GB Pant University of Agriculture & Technology.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- Scrosati, B., Garche, J. (2010) Journal of Power Sources, 195(9), 2419-2430.
- Srivastava, N. K., Mehra, R. M. (2008) Journal of Applied Polymer Science, 109(6), 3991-3999.
- Li, J., & Gao, F. (2009) Journal of Power Sources, 194(2), 1184-1193.
- Ürkmez, G., Sarı, B., Ünal, H. İ. (2011) Journal of Applied Polymer Science, 121(3), 1600-1609.
- Senthilkumar, P., Jayasankar, S., Sateesh, V. L., Kamaleshaiah, M. S., Dayananda, G. N. (2013) Smart Materials and Structures, 22(9), 095025.
- Tebyetekerwa, M., Yang, S., Peng, S., Xu, Z., Shao, W., Pan, D Zhu, M. (2017) Electrochimica Acta, 247, 400-409.
- Danaee I, Jafarian M, Forouzandeh F, Gobal F, Mahjani MG. (2009) International Journal Hydrogen Energy, 34:859–69.
- Conway BE. (1999) Dordrecht/New York: Kluwer Academic Publishers/Plenum Press.
- Lin, W.-D.; Chang, H.-M.; Wu, R.-J. (2013) Sensors and Actuators B: Chemical 181, 326–331.
- Farahani, H., Wagiran, R., Hamidon, M. N. (2014). Sensors, 14(5), 7881-7939.
- Steinhauser, J., Meyer, S., Schwab, M., Faÿ, S., Ballif, C., Kroll, U., Borrello, D. (2011) Thin Solid Films, 520(1), 558-562.
- Zhang, L., Tarascon, J. M., Sougrati, M. T., Rousse, G., Chen, G. (2015) Journal of Materials Chemistry A, 3(33), 16988-16997.
- Yang, W. S., Noh, J. H., Jeon, N. J., Kim, Y. C., Ryu, S., Seo, J., Seok, S. I. (2015) Science 348(6240), 1234-1237.
- Wei, Z., Zheng, X., Chen, H., Long, X., Wang, Z., Yang, S. (2015) Journal of Materials Chemistry A, 3(32), 16430-16434.
- Yang, J., Kelly, T. L. (2017) Inorganic chemistry, 56(1), 92-101.
- Zhao, L., Kerner, R. A., Xiao, Z., Lin, Y. L., Lee, K. M., Schwartz, J., Rand, B. P. (2016) ACS Energy Letters, 1(3), 595-602.
- Bohara, R. A., Throat, N. D., Mulla, N. A., Pawar, S. H. (2017) Applied Biochemistry and Biotechnology, 182(2), 598-608.
- Park, H. K., Kim, S. M., Lee, J. S., Park, J. H., Hong, Y. K., Hong, C. H., Kim, K. K. (2015) Synthetic Metals, 203, 127-134.
- Nayak, D., Choudhary, R. B. (2019) Optical Materials, 91, 470-481.
- Sanjinés, R., Abad, M. D., Vâju, C., Smajda, R., Mionić, M., Magrez, A. (2011) Surface and Coatings Technology, 206(4), 727-733.
- Wen, Z., Wu, M., Lin, Y., Yang, L., Lin, J., Cen, P. (2014) Microbial Cell Factories, 13(1), 92.
- Zhang, X., Tang, Y., Zhang, F., Lee, C. S. (2016) Advanced Energy Materials, 6(11), 1502588.
- Li, D., Jiang, Y., Wu, Z., Chen, X., Li, Y. (2000) Sensors and Actuators B: Chemical, 66(1-3), 125-127.
- Shih, W. P., Tsao, L. C., Lee, C. W., Cheng, M. Y., Chang, C., Yang, Y. J., Fan, K. C. (2010) Sensors, 10(4), 3597-3610.
- Kawde, A. N., Baig, N., Sajid, M. (2016) RSC Advances, 6(94), 91325-91340.
- Rikhari, R., Saklani, B., Bisht, A., Mehtab, S., Zaidi M.G.H. (2019) Sensor Letters, 17, 511-515.
- Ji, J., Zhang, L. L., Ji, H., Li, Y., Zhao, X., Bai, X., Ruoff, R. S. (2013) ACS Nano, 7(7), 6237-6243.
- Gopalakrishnan, M., Srikesh, G., Mohan, A., Arivazhagan, V. (2017) Applied Surface Science, 403, 578-583.
- Jadhav, K., Deore, S., Dhamecha, D., Hr, R., Jagwani, S., Jalalpure, S., Bohara, R. (2018) ACS Biomaterials Science and Engineering, 4(3), 892-899.
- Sengupta, R., Bhattacharya, M., Bandyopadhyay, S., Bhowmick, A. K. (2011) Progress in Polymer Science, 36(5), 638-670.
- Rita, Mehtab, S., ZAIDI, M.G.H., Singhal, K., Arya, B., Siddiqui, T.I., (2019) Material Science Research India 16 (2), 97-102.
- Cai, Z. J., Zhang, Q., Song, X. Y. (2016) Electronic Materials Letters, 12(6), 830-840.
- Alshammari, A. S. (2018) Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications, 331-360.
- Zhang, J., Yu, Y., Liu, L., Wu, Y. (2013) Nanoscale, 5(7), 3052-3057.
- Raj, R. P., Ragupathy, P., Mohan, S. (2015) Journal of Materials Chemistry A 3 (48), 24338-24348.
- Wang, H., Lin, J., Shen, Z. X. (2016) Journal of Science: Advanced Materials and Devices, 1(3), 225-255.
- Joshi, L., Prakash, R. (2011) Materials Letters, 65(19-20), 3016-3019.
- Kumar, A., Pandey, A. C., Prakash, R. (2012) Catalysis Science & Technology, 2(12), 2533-2538.
- Inzelt, G. (2018) Journal of Electrochemical Science and Engineering, 8(1), 3-37.
- Zhou, W., Xu, J. (2017) Polymer Reviews, 57(2), 248-275.
- Ramesan, M. T., Anjitha, T., Parvathi, K., Anilkumar, T., Mathew, G. (2018) Advances in Polymer Technology, 37(8), 3639-3649.
- Elango, M., Deepa, M., Subramanian, R., Musthafa, A. M. (2017) Journal of Alloys and Compounds, 696, 391-401.
- Shakir, I., Ali, Z., Bae, J., Park, J., Kang, D. J. (2014) Nanoscale, 6(8), 4125-4130.
- Stejskal, J., Mrlík, M., Plachý, T., Trchová, M., Kovářová, J., Li, Y. (2017) Reactive and Functional Polymers, 120, 30-37.
- Joshi, P., Mehtab, S., Zaidi, M.G.H., Tyagi, T., Bisht, A. (2020) Journal of Nanostructure in Chemistry, 10(1), 33-45.
- Sharma, S., Joshi, P., Mehtab, S., Zaidi, M.G.H., Singhal, K., Siddiqi, T.I. (2020) Journal of Analysis and Testing, 1-10
- Phasuksom, K., Sirivat, A. (2016) Synthetic Metals, 219, 142-153.
- Khan, M. J., Yadav, A. K., Mathew, L. (2017) Renewable and Sustainable Energy Reviews, 76, 577-607.
- Spriggs, G. E. (1995) International Journal of Refractory Metals and Hard Materials, 13(5), 241-255.
- Tallo, I., Thomberg, T., Kontturi, K., Jänes, A., Lust, E. (2011) Carbon, 49(13), 4427-4433.
- Joshi, I. L. A., Khati, K., Bisht, A., Rikhari, R., Mehtab, S., Zaidi, M. G. H. (2018) International Journal of Chemical Studies, 6(3), 3600-3603.
- Tomer, V. K., Duhan, S. (2016) Sensors and Actuators B: Chemical, 223, 750-760.
- Han, Y., Meyer, S., Dkhissi, Y., Weber, K., Pringle, J. M., Bach, U. Cheng, Y. B. (2015) Journal of Materials Chemistry A, 3(15), 8139-8147.
- Raza, M. A., Westwood, A., Brown, A., Hondow, N., Stirling, C. (2011) Carbon, 49(13), 4269-4279.
- Sehgal, P., Narula, A. K. (2015) Colloid and Polymer Science, 293(9), 2689-2699.
- Tebyetekerwa, M., Wang, X., Wu, Y., Yang, S., Zhu, M., Ramakrishna, S. (2017) Journal of Materials Chemistry A, 5(40), 21114-21121.
- Jayakrishnan, P., Ramesan, M. T. (2017) Journal of Inorganic and Organometallic Polymers and Materials, 27(1), 323-333.
- Cai, Z., Yang, G. (2010) Synthetic Metals, 160(17-18), 1902-1905.
- Zhijiang, C., Qing, Z., Cong, Z., Xianyou, S., Yuanpei, L. (2017) Synthetic Metals, 231, 15-18.
- Bhagat, D. J., Dhokane, G. R. (2015) Chemical Physics Letters, 619, 27-31.
- Bhagat, D. J., Dhokane, G. R. (2016) Journal of Materials Science: Materials in Electronics, 27(11), 11790-11797.
- Drakonakis, V. M., Savva, A., Kokonou, M., Choulis, S. A. (2014) Solar Energy Materials and Solar Cells, 130, 544-550.
- Li, D., Jiang, Y., Wu, Z., Chen, X. and Li, Y., (2000) Sensors and Actuators B: Chemical, 66(1-3): 125-127.
- Khalil, K. M., Makhlouf, S. A. (2008) Sensors and Actuators A: Physical, 148(1), 39-43.
- Wang, G., Morrin, A., Li, M., Liu, N., Luo, X. (2018) Journal of Materials Chemistry B, 6(25), 4173-4190.
- Ramani, V., Kunz, H. R., Fenton, J. M. (2005) Electrochimica Acta, 50(5), 1181-1187.
- Hoffmann, S., Koehl, M. (2014) Progress in Photovoltaics: Research and Applications, 22(2), 173-179.
- Phasuksom, K., Prissanaroon-Ouajai, W., Sirivat, (2018) Sensors and Actuators B: Chemical, 262, 1013-1023.
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. (2016) Carbon, 105, 23-32.
- Lin, W. D.; Liao, C.T.; Chang, T. C.; Chen, S.H.; Wu, R. J. (2015) Sensors and Actuators B: Chemical, 209, 555–561.
- Li, X.; Chen, X.; Chen, X.; Ding, X.; Zhao, X. (2018) Materials Chemistry and Physics 207, 135-140.
- Kafy, A.; Akther, A.; Shishir, M.I.R.; Kim, H.C.; Yun, Y.; Kim, J. (2016) Sensors and Actuators A: Physics, 247, 221–226.
Views: 1,514
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Pragati Joshi1, Bhagwati Arya1, M.G.H. Zaidi1*
, Pragati Joshi1, Bhagwati Arya1, M.G.H. Zaidi1* and Tanveer Irshad Siddiqui2
and Tanveer Irshad Siddiqui2
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal