Hepatorenal Effects of Silver Nanoparticles in In-Vivo Postnatal Model of Toxicity and in HepG2 Cell Line
Mahmoud M. Elalfy1* , Mamdouh Abouelmagd1, Eman A. Abdelraheem1 and Mona G. El-hadidy2
, Mamdouh Abouelmagd1, Eman A. Abdelraheem1 and Mona G. El-hadidy2
1Forensic and toxicology department, faculty of veterinary medicine, Mansoura University, Egypt
2Medical physiology department faculty of medicine, Mansoura university, Egypt
Corresponding Author Email: mahmoudelalfy@mans.edu.eg
DOI : http://dx.doi.org/10.13005/msri/170108
Article Publishing History
Article Received on : 01 April 2020
Article Accepted on : 30 April 2020
Article Published : 01 May 2020
Plagiarism Check: Yes
Reviewed by: Łukasz Łopusiewicz
Second Review by: Ajoy Kumer
Final Approval by: Prof. K. M. Garadkar
Article Metrics
ABSTRACT:
Silver nanoparticles (Ag-NPs) had many uses in medicine, household and industry. To better understand the postnatal toxicity of Ag-NPs in lactating female rats and its offspring’s, 18 female rats after delivery were divided into three groups and dams received orally the AG-NPs at doses of 0, 50, 100 ppm daily for 21 days. After the end of treatment, all rats were euthanized and blood and tissues were separated for evaluation of biochemical and histopathology in dams and its pups. The Ag-NPs had no effect on the dam's weight while the reduction of rats’ pups weight was noticed after first week only after the treatment. Notably, Ag-NPs had toxic effects in rat’s pups, as well as its dam with evidence of elevation of liver enzymes, urea, creatinine and reduction of serum protein, albumin and globulin and considered the first report explained the toxicity in the rat’s pups. Moreover, rats' pups revealed histopathological changes in liver and kidney as well as its dams. Notably, the nano-silver is considered cytotoxic for HepG2 cell line as well as mouse liver cell line. In conclusions, the Ag-NPs considered toxic in offspring as well as dams and had immunosuppressive effects in the postnatal model of toxicity as well as cytotoxicity to hepatic cells lines.
Illustrated graphical abstract of Ag NPs toxicity in female albino rats
KEYWORDS:
Ag-NPs; Lactating Female Rats Offspring; Liver and Kidney
Copy the following to cite this article:
Elalfy M. E, Abouelmagd M, Abdelraheem E. A, El-hadidy M. G. Hepatorenal Effects of Silver Nanoparticles in In-Vivo Postnatal Model of Toxicity and in HepG2 Cell Line. Mat. Sci. Res. India; 17(1).
|
Copy the following to cite this URL:
Elalfy M. E, Abouelmagd M, Abdelraheem E. A, El-hadidy M. G. Hepatorenal Effects of Silver Nanoparticles in In-Vivo Postnatal Model of Toxicity and in HepG2 Cell Line. Mat. Sci. Res. India; 17(1). Available from: https://bit.ly/2KRvcPX
|
Background
Ag-NPs are promising material for drug, antiseptics, household and anticancer therapy.1 Its side effects are lasting but still have no approval of widespread uses as its toxicity for cells of human, animal and environment are detected.2, 3
There were many studies on Ag-NPs on prenatal studies on both pups and dams in different animals’ species. These studies evoked that Ag-NPs are widely distributed in all tissues and can cross the placental barrier and transfer to pups through milk suckling.4-6
The rout of administration of Ag-NPs is considered a determinant factor in severity of toxic effects of Ag-NPs as it previously recorded that 4 to 18 % only absorbed through GIT in mammals while it can absorb from other routs with high levels.7 Even the low-level Ag-NPs reach blood circulation after oral administration, still evidence that Ag-NPs were distributed in different tissues of rats’ dam.8 Also type of Ag-NPs could also another factor affecting its toxicity as chemical synthesized Ag-NPs expect to have more toxic complications than that produced by green means by use of plants extracts.9
The mechanism of toxicity of oral exposure Ag-NPs to rat dams and pups may be due to induction of oxidative stress and apoptosis in the liver of their offspring.10 The rationale of this study to investigate the cytotoxicity of Ag-NPs in female rats and its pups in postnatal model of toxicity
Materials and Methods
Laboratory Animals
Female albino rats obtained from the experimental unit, Faculty of veterinary medicine, Zagazig University, weighted from 180 to 200 gram. Animals were apparently healthy and housed in plastic cages containing wood shaving as a bedding material. Animals accommodated for 2 weeks before the experiment and maintained on a balanced ration also feed and water given ad libitum. We follow all guidelines of animal ethics in faculty of veterinary medicine, Mansoura University, Egypt.
Tested chemicals and cell culture
Nano-Silver, its size less than 100 nm, (USA, MKCD7246) and HepG2 cell line were purchased from Sigma Aldrich.
Experimental grouping and design
Eighteen pregnant female rats divided into three groups each one contained six rats weighed 150 to 200 g and kept until delivery. In the current study, Silver Nanoparticles (Sigma Aldrich, USA) given orally, dissolved in di-ionized water, at dose level 0, 50, 100 ppm ( equivalent to 1/50 and 1/100 of the LD50 recorded earlier11 on first day of lactation till 21 days and control group intubated with di-ionized water as control. Dams weighted before dosing to maintain constant dose throughout the experimental period. On 21st day of lactation, all rats were sacrificed by over dose of thiopental, blood of dams and pups were collected for serum separation and different tissue samples were collected. Tissues of pups were separated and fixed in buffered formalin for histopathological examination.
Histopathological Examination
Different tissues from dams and pups were fixed in 10% neutral buffered formalin. Fixed tissue where procedures were described before in detail for classical eosin and hematoxylin staining, imaging and reading for pathology detection.12
Biochemical analysis
Sera of all dams and pups of treated or control groups were tested for liver and kidney function tests for evaluation the postnatal toxicity of AG-NPs
Cytotoxicity of Silver Nanoparticles on of HepG2 cells
The stock solution of Ag NPs (100 mmol/L) was prepared in di-ionized water and stored at 4°C. Working solutions were prepared by dissolving the stock solution in the culture medium. We exposed HepG2 cells and the primary mouse liver cell line to different concentrations (0, 10,100,200 ppm/mL) of Ag NPs for 24 hours to determine its toxic effects. The HepG2 cells and mouse liver primary cell line were cultured according to methods described earlier.13
Cell viability and oxidative stress tests
The viability of cells was detected by quantification of formed formazan salt. In this regard, HepG2 cells (2 ×105 cells) were seeded in 96-well plates. Later 24 h, the medium solution was changed with other medium containing different concentrations (0,10,100,200 ppm) of silver nanoparticles and di-ionized water were added for 24. MTT (50 µg/mL) was added to each well. After 4 h incubation at 37 °C, the later solution was discarded and formed formazan crystals was dissolved in DMSO (100 µL). The color developed was measured at 570 nm using a multiplate reader (Synergy HT, Bio-Tek, Winooski, Vermont). Additionally, the cell extract was centrifuged (10000 g, 10 min, 4ºC) and supernatant was used for oxidative stress assays such as glutathione (GSH), superoxide dismutase (SOD), and MDA.
Statistical Analysis was carried out using t-test and Oneway Annova. and P<0.05 was considered significant (Spss version 13) (14).
Results
Dams and Pups weights
There was no effect on body weight of rat dams while the pups had reduced body weight only after one week of treatment when compared to control group. (Table 1)
Table 1: showed the postnatal effect of AgNPs on body weight of rat pups during the treatment
| |
First week of treatment
|
Second week of treatment
|
Third week of treatment
|
|
Group 1
|
12.80±0.46212
|
18.52±0.76c
|
34.39±1.032a
|
|
Control
|
|
|
|
|
Group 2
|
12.52±0.28196
|
23.05±0.51b
|
35.59±0.77a
|
|
Group 3
|
15.30±0.27750
|
28.19±0.60a
|
36±0.83a
|
a, b, c significant at ≤ 0.05
Biochemical Effects of Silver Nanoparticles on Dams and its Pups
Here, we are the first report that recorded the effects of the toxicity of Ag-NPs in rat pups in postnatal model of toxicity especially biochemical alteration in the liver and kidney tissue. The hepatorenal toxicity of Ag-NPs was based on significant increase of activity of liver enzymes (AST, ALT) and levels of creatinine, urea and cholesterol in dose dependent manner when compared to control groups. Also, it enhanced the reduction of total protein, albumin and albumin/globulin ratio when compared to the control group especially at low dose of silver nanoparticles treatment. Additionally, AG-NPs could have immunosuppression effects as it reduced the globulin level (table).
Table 2: The hepatorenal effects of oral administration of Ag-NPs treatment in postnatal toxicity in rat dams
|
Urea
|
Creatinine (mg/dL)
|
Cholesterol (mmol/L)
|
Urea (mmol/L)
|
Creatinine (mg/dL)
|
Cholesterol (mmol/L)
|
|
Group 1
|
7.80±1.511b
|
29.89±2.06b
|
79.73±2.70b
|
39.4155±2.87b
|
4.23±.33b
|
|
Group 2
|
26.67±2.42a
|
47.88±5.08a
|
98.81±5.96a
|
44.01±1.05ab
|
4.4977±.27b
|
|
Group 3
|
28.49±3.126a
|
52.92±5.28a
|
105.07±8.67a
|
45.46±.97a
|
6.1482±.74a
|
a, b, c significant at ≤ 0.05
The fetal toxicity of Ag-NPs in rat pups was evidenced by increased activity of liver enzymes (ALT, AST) and levels of urea, creatinine, and cholesterol in significant manner and dose dependent effect when compared to control groups. Also, the Ag-NPs had no effect on total protein and globulin levels of pups. Notably, albumin and albumin/globulin ration were reduced in significant manner when compared to control groups.
Table 3: show the effects of oral administration of AG-NPs treatments in protein metabolism of rat dams
| |
Total protein
|
Albumin
|
Globulin
|
Albumin / globulin ratio
|
|
Group 1
|
11.41±0.39162a
|
5.85±0.22369a
|
5.56±0.45
|
1.11±0.09
|
|
Group 2
|
9.36± 0.45469b
|
4.90±0.23192b
|
4.45±0.50
|
1.18±0.20
|
|
Group 3
|
10.34±0.60785ab
|
5.43±0.10869ab
|
4.90±0.58
|
1.20±0.14
|
a, b, c significant at ≤ 0.05
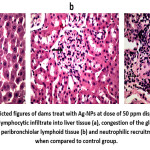
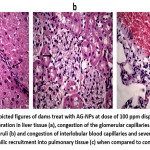
Pathological finding of Ag-NPs treatment in rat dams and its pups. The group treated with Ag-NPs at dose of 50 ppm shown mild lymphocytic infiltrate into hepatic tissue, congestion of the glomerular capillaries and proliferation of peribronchiolar lymphoid tissue and neutrophilic recruitment. While group treated with 100ppm of AG-NPs displayed hepatic intralobular fibroblastic proliferation, congestion of the glomerular capillaries and proliferation of the renal glomeruli and congestion of interlobular blood capillaries and neutrophilic recruitment into pulmonary tissue (figure 1, 2).
Figure 1: The depicted figures of dams treat with Ag-NPs at dose of 50 ppm displayed histological alteration as mild lymphocytic infiltrate into liver tissue (a), congestion of the glomerular capillaries and proliferation of peribronchiolar lymphoid tissue (b) and neutrophilic recruitment of lung tissue (c) when compared to control group.
Figure 2: The depicted figures of dams treat with AG-NPs at dose of 100 ppm displayed intralobular fibroblastic proliferation in liver tissue (a), congestion of the glomerular capillaries and proliferation of the renal glomeruli (b) and congestion of interlobular blood capillaries and sever hemorrhage and neutrophilic recruitment into pulmonary tissue (c) when compared to control group.
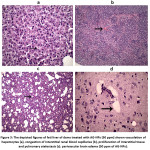
Notably, silver nanoparticle secreted in milk and enhanced histopathological changes at dose of 50 ppm in rat pups such as perivascular edema in brain parenchyma, neutrophilic recruitment into the renal glomeruli, local necrosis of hepatocytes and proliferation of interstitial tissue with pulmonary atelectasis and hemorrhage. While the AG-NPs at 100 ppm induced perivascular edema in brain parenchyma, congestion of interstitial blood capillaries of renal tissue, vacuolation of hepatocytes and proliferation of interstitial tissue and pulmonary atelectasis (figure 3).
Figure 3: The depicted figures of feti liver of dams treated with AG-NPs (50 ppm) shown vacuolation of hepatocytes (a), congestion of interstitial renal blood capillaries (b), proliferation of interstitial tissue and pulmonary atelectasis (c). perivascular brain edema (50 ppm of AG-NPs).
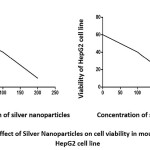
Notably, the viability percentage measured by MTT assay on primary liver cell of mice or HepG2 cells exposed to 0, 1, 10, 100 and 200 ppm of AgNPs for 24 h displayed a dose-response pattern as shown in Figure 4. Also silver nanoparticles enhanced oxidative stress in HepG2 cells based on increase MDA and reduction of GSH and GST (table 4).
Figure 4: Pattern effect of Silver Nanoparticles on cell viability in mouse liver cell line and HepG2 cell line
Table 4: Showed effect of oxidative stress in HepG2 cell line
|
MDA
|
SOD
|
GSH
|
Concentration of Silver Nanoparticles (ppm)
|
|
nmol/g tissue
|
U/g tissue
|
mg/g tissue
|
|
14.97±0.93c
|
8.42±0.054a
|
20.69±1.095a
|
0
|
|
17.97±0.63c
|
7.42±0.035a
|
18.69±1.05a
|
10
|
|
53.97±0.93b
|
4.42±0.054b
|
9.69±1.03b
|
100
|
|
75.96±1.90a
|
2.08±0.53b
|
5.91±1.042b
|
200
|
a, b, c significant at ≤ 0.05
Discussion
There was several evidence that AG-NPs presence in milk of lactating rats1 or mice2 was reported previously after treatment with different routes of administration.3-5
In the current research, there was no effect of silver nanoparticles on body weight of dams while the pups had effect on body weight only after one week of treatment when compared to the control group (table 1). In this regard, there was no difference on pup’s weight among treated groups by silver nanoparticles reported by Rashno et al.,7, 15
The histopathological changes in rat pups such as edema in the brain and liver necrosis (figure 3) recorded also in several studies after oral exposure to silver nanoparticles.7, 8 Also, Amiri et al.,8 investigated the brain toxicity of Ag-NPs on pregnant female mice and it’s offspring during and after pregnancy. Moreover, the exposure to AgNPs may enhanced apoptosis and necrosis via the intrinsic pathway in the offspring tissues of rats treated with 25 and 100 ppm of Ag-NP for 21 days.9 The pathological alteration was prominent in the dam (figure 1,2) as well as in its offspring (figure 3) due to an immature immune system especially in early postnatal period, lack of protective enzymes, high metabolic rate, and low infiltration rate.10, 11 Also, another evidence recorded presence of distribution of Ag-NPs in kidney, lung and liver of rat’s pups5 and this accumulation of AG-NPs could release Ag ions that are responsible for toxicity induction 16 and similar studies revealed the reduction of total protein and albumin (table 3) after silver nanoparticles treatments.17, 18 While few studies reported that Ag-NPs elevated the total protein in non–significant manner as silver nanoparticle binding with albumin and retain its presence in serum after exposure to high dose.19, 20 Moreover, the oral administration of Ag-NPs considered less toxic than intraperitoneal treatment as only 4-18 % of Ag-NPs were absorbed after oral treatments21 So, the toxicity of silver nanoparticles could result in damage of liver parenchyma based on the reduction of serum protein and elevation of liver enzymes (figure 2)13, 14 as well as our finding in rat’s pups as they had immature liver. Notably, the silver nanoparticles had cytotoxic effects on HepG2 and primary mouse liver cell line19, 20 thorough enhancement of oxidative stress and reduction of antioxidants (figure 4 and table 4).
In conclusion, the Ag-NPs considered to be toxic in offspring as well as dams and had immunosuppressive effects in the postnatal model of toxicity. Also it had a toxic effect in HepG2 cell line mediates by oxidative stress.
Conflict of Interest
All author declares that no conflict of interest
Author Contributions
Mahmoud elalfy, Eman Abdelraheem and Mona El-hadidy designed, carried out the experiment, analysis, write and approved the submission. Mamdouh abouelmagd supervised and approved the submission.
Acknowledgment
We thank dr Mohamed fawzy for grateful help in pathological evaluation of all stained tissue sections of organs of treated groups.
Funding Source
This research received no specific grant from any funding agency.
References
- Burdușel A-C, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials. 2018;8(9):681.
- Panyala NR, Peña-Méndez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? Journal of Applied Biomedicine (De Gruyter Open). 2008;6(3).
- Quadros ME, Marr LC. Environmental and human health risks of aerosolized silver nanoparticles. Journal of the Air & Waste Management Association. 2010;60(7):770-81.
- Takeda K, Suzuki K-i, Ishihara A, Kubo-Irie M, Fujimoto R, Tabata M, et al. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. Journal of Health science. 2009;55(1):95-102.
- Kulvietis V, Zalgeviciene V, Didziapetriene J, Rotomskis R. Transport of nanoparticles through the placental barrier. The Tohoku journal of experimental medicine. 2011;225(4):225-34.
- Melnik E, Demin V, Demin V, Gmoshinski I, Tyshko N, Tutelyan V. Transfer of silver nanoparticles through the placenta and breast milk during in vivo experiments on rats. Acta Naturae (англоязычная версия). 2013;5(3 (18)).
- Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, et al. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3(2):109-38.
- Brohi RD, Wang L, Talpur HS, Wu D, Khan FA, Bhattarai D, et al. Toxicity of nanoparticles on the reproductive system in animal models: a review. Frontiers in pharmacology. 2017;8:606.
- Roy N, Gaur A, Jain A, Bhattacharya S, Rani V. Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environmental toxicology and pharmacology. 2013;36(3):807-12.
- Fatemi M, Moshtaghian J, Ghaedi K. Effects of silver nanoparticle on the developing liver of rat pups after maternal exposure. Iranian journal of pharmaceutical research: IJPR. 2017;16(2):685.
- Maneewattanapinyo P, Banlunara W, Thammacharoen C, Ekgasit S, Kaewamatawong T. An evaluation of acute toxicity of colloidal silver nanoparticles. Journal of Veterinary Medical Science. 2011:1106220557-.
- Bancroft John D, Alan S. Theory and Practice of histological techniques. Churchill Livingstone Elsevier. 2002;7.
- Snedecor G, Cochran W. Statistical methods., 8th edn.(Iowa State University Press: Ames, IA). 1989.
- Morishita Y, Yoshioka Y, Takimura Y, Shimizu Y, Namba Y, Nojiri N, et al. Distribution of silver nanoparticles to breast milk and their biological effects on breast-fed offspring mice. ACS nano. 2016;10(9):8180-91.
- Lee Y, Choi J, Kim P, Choi K, Kim S, Shon W, et al. A transfer of silver nanoparticles from pregnant rat to offspring. Toxicological research. 2012;28(3):139-41.
- Rashno M, Fatemi Tabatabaei SR, Khaksary Mahabady M, Ghaderi S. Maternal exposure to silver nanoparticles in mice: effects on dams’ reproductive performance and pups’ neurobehavioral ontogeny. Anatomical Sciences Journal. 2014;11(1):41-52.
- Amiri S, Yousefi-Ahmadipour A, Hosseini M-J, Haj-Mirzaian A, Momeny M, Hosseini-Chegeni H, et al. Maternal exposure to silver nanoparticles are associated with behavioral abnormalities in adulthood: Role of mitochondria and innate immunity in developmental toxicity. NeuroToxicology. 2018;66:66-77.
- Rastogi RP, Sinha RP. Apoptosis: molecular mechanisms and pathogenicity. 2010.
- Oskarsson A, Hallén IP, Sundberg J, Grawé KP. Risk assessment in relation to neonatal metal exposure. Analyst. 1998;123(1):19-23.
- Ema M, Okuda H, Gamo M, Honda K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reproductive Toxicology. 2017;67:149-64.
- Sulaiman FA, Adeyemi OS, Akanji MA, Oloyede HOB, Sulaiman AA, Olatunde A, et al. Biochemical and morphological alterations caused by silver nanoparticles in Wistar rats. Journal of Acute Medicine. 2015;5(4):96-102.
- Adeyemi OS, Adewumi I. Biochemical evaluation of silver nanoparticles in wistar rats. International scholarly research notices. 2014;2014.
- POORHAMZE M, GHOLAMI MZ, Saidijam M, ASARI MJ, Alizadeh Z. The effect of silver nanoparticles on the biochemical parameters of liver function in serum, and the expression of caspase-3 in the liver tissues of male rats. 2016.
- Parang Z, Moghadamnia D. Effects of silver nanoparticles on the functional tests of liver and its histological changes in adult male rats. Nanomedicine Research Journal. 2018;3(3):146-53.
- Elalfy MM, Abdraheem EE, Abouelmagd M. Effect of oral administration of silver nanoparticles on blood parameters and bone marrow cells of female albino rats.
Views: 740
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Mamdouh Abouelmagd1, Eman A. Abdelraheem1 and Mona G. El-hadidy2
, Mamdouh Abouelmagd1, Eman A. Abdelraheem1 and Mona G. El-hadidy2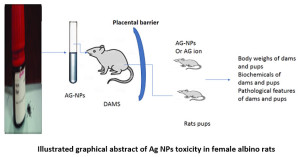
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal