Complex Impedance Spectroscopic Studies of PMMA(80,70,60,50) : PC(10,20,30,40): PVP(10): LiClO4(5) Polymer Solid Electrolyte Systems
R. Swarnalatha1 , A. Sadananda Chary2, Venkata Ramana Jeedi 3, Yalla Mallaiah 4 and S. Narender Reddy5
, A. Sadananda Chary2, Venkata Ramana Jeedi 3, Yalla Mallaiah 4 and S. Narender Reddy5
1Department of Physics, University College of Engineering (A), Osmania University, Hyderabad, Telangana, India.
2Department of Physics, University College of Science, Osmania University, Hyderabad, Telangana, India.
3Department of Physics, B V Raju Institute of Technology, Narsapur, Medak Dist, Telangana, India.
4Department of Physics, University P.G College Secunderabad, Osmania University, Hyderabad, Telangana, India.
5Department of Physics, Stanley College of Engineering and Technology for Women, Abids, Hyderabad, Telangana, India.
Corresponding Author E-mail: swarnalatha424@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170310
Article Publishing History
Article Received on : 2-November-2020
Article Accepted on : 23-Dec-2020
Article Published : 26 Dec 2020
Plagiarism Check: Yes
Reviewed by: Dr. Paroma Arefin paroma
Second Review by: Dr. Arun Sharma
Final Approval by: Dr. Nagaraja K K
Article Metrics
ABSTRACT:
Polymer solid electrolytes PMMA(80,70,60,50): PC(10,20,30,40):PVP(10):LiClO4(5) were synthesized according to stoichiometric ratios using solution cast method. FTIR study confirms the good complexation among the constituent materials in the polymers matrix. Complex impedance and electric modulus studies are carried out and explained. From cole-cole plots, maximum decrement of resistance is observed at a threshold ratio 70Wt% PMMA: 20Wt% PC: 10Wt% PVP: 5Wt% LiClO4 and this could be due to the high mobility of the Li+ ion in the polymer network due to the plasticizer. The plasticizer plays an important role in decreasing the viscosity of the system, which in turn favors the mobility of segmental motion of polymer network and fast ion motion in the polymer. Real and Imaginary electric modulus spectra show the presence of relaxation peaks and confirm that the polymer solid electrolyte is an ionic conductor.
KEYWORDS:
Bulk Resistance; Electric Modulus; Ionic Conductor; Segmental Motion
Copy the following to cite this article:
Swarnalatha R, chary A. S, Jeedi V. R, Mallaiah Y, Reddy S. N. Complex Impedance Spectroscopic Studies of PMMA(80,70,60,50) : PC(10,20,30,40): PVP(10): LiClO4(5) Polymer Solid Electrolyte Systems. Mat. Sci. Res. India; 17(3).
|
Copy the following to cite this URL:
Swarnalatha R, chary A. S, Jeedi V. R, Mallaiah Y, Reddy S. N. Complex Impedance Spectroscopic Studies of PMMA(80,70,60,50) : PC(10,20,30,40): PVP(10): LiClO4(5) Polymer Solid Electrolyte Systems. Mat. Sci. Res. India; 17(3). Available from: https://bit.ly/38Fc5UR
|
Introduction
Recently a rapid increase in the application of electronic devices leading to the development of the necessity of high energy stored secondary batteries1. Mostly Li-ion-based batteries are available in a liquid state, but they possess leakage problems which may cause fire and explosion, thereby raising safety issues2,3. These problems have been minimized in polymer-based solid electrolytes and more research has been carried out and reported in solid polymer electrolyte membranes, namely PEO-PMMA-LiClO4-Silica Aerogel4, PMMA-LiBF45, PEO-LiClO4-ZnO6, Al2O3-EC-PEO-LiCF3SO37, EC-PVC-LENR508, PMMA-LiClO4-Clay9, (PC+DEC)-LiClO4-PMMA10, PMMA-LiClO4-EC-PC-PEG-DMC11, etc.. In the present work, we have studied the report the FTIR characterization, Cole-Cole plots and electric modulus of solid blend polymer electrolyte systems PMMA(80,70,60,50): PC(10,20,30,40): PVP(10): LiClO4(5).
Experiment
PMMA ( Mw ̴1.2×105 ), PVP(Mw ̴ 3.6×105), PC (Mw ̴ 105), DMF (N-N dimethylformamide), and LiClO4 chemicals were procured from Sigma Aldrich India. Solid polymer electrolytes were synthesized with varying PMMA (Polymethyl methacrylate) and PC (Polycarboxylic), keeping the remaining two chemicals PVP (Polyvinylpyrrolidone) LiClO4 (Lthium perchlorate) kept constant using the solution casting method. Thereafter these were designated as PPSE1, PPSE2, PPSE3, and PPSE4, respectively, as shown in table-1. The above chemicals were dissolved in 30 ml of DMF and stirred for nearly 24 hours at ambient temperature until a homogeneous solution was formed; then the polymer mixture was poured into a petri dish and kept in a vacuum oven for about 70oC. A dried polymer film was formed after 48 hours.
Table 1: Designation of the polymer matrixes used in this work
|
Sl.No.
|
Polymer Matrix
|
Polymer Name
|
|
1
|
80Wt% PMMA : 10Wt% PC : 10Wt% PVP : 5Wt% LiClO4
|
PPSE-1
|
|
2
|
70Wt% PMMA : 20Wt% PC : 10Wt% PVP : 5Wt% LiClO4
|
PPSE-2
|
|
3
|
60Wt% PMMA : 30Wt% PC : 10Wt% PVP : 5Wt% LiClO4
|
PPSE-3
|
|
4
|
50Wt% PMMA : 40Wt% PC : 10Wt% PVP : 5Wt% LiClO4
|
PPSE-4
|
Results and Discussion
Complex Impedance Plots
Complex impedance spectroscopy studies are carried out for different polymer solid electrolytes. Figure 1 shows the cole-cole plots at different temperatures for PPSE1, PPSE2, and PPSE3 solid polymer systems, respectively. The intercepts of the semicircle on the real axis give the bulk resistance (Rb) of the sample when a plot is drawn between Z’ and Z” at a given temperature. The conductivity of the sample is evaluated at a given temperature by using
σ= t ̷ ARb
Where ‘t’ is the thickness and ‘A’ is the area of the cross-section of the sample. From Cole-Cole plots, it has been observed that the magnitude of the bulk resistance (Rb) decreases with an increase in temperature in all these three polymer matrices6,9. The maximum decrement of resistance is observed at a threshold ratio 70Wt% PMMA: 20Wt% PC: 10Wt% PVP: 5Wt% LiClO4 (PPSE2) and this could be due to the high mobility of the Li+ ion in the polymer network.
Figure 1: (a), (b), and (c) Cole-Cole plots of the samples PPSE1, PPSE2, and PPSE3 at different temperatures.
Electric Modulus
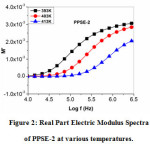
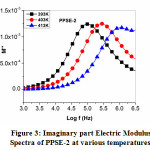
The real (M΄) and imaginary (M΄΄) electric modulus spectra as a function of temperature at different frequencies for PPSE2 have been shown in figures 2 and 3, respectively. Real electric modulus spectra show that there is dispersion at the high-frequency region and a long tail with ‘0’ magnitude at low frequency, indicating the presence of large capacitance associated with the electrode. Imaginary modulus spectra in figure 6 show the peak at different temperatures. The left and right side of the peak gives information about the conduction process(long-range mobility of charge carriers) and the relaxation process of the conducting ions(short-range mobility of charge carriers)12,14. There is a shift in the asymmetric peaks, which can be understood that there exists a correlation between the mobility of different charge carriers. The presence of relaxation peaks confirms that the polymer solid electrolyte is an ionic conductor13,14.
Figure 2: Real Part Electric Modulus Spectra of PPSE-2 at various temperatures.
Figure 3 Imaginary part Electric Modulus Spectra of PPSE-2 at various temperatures.
FTIR
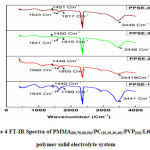
Figure 4 depicts the FTIR spectra of four polymer electrolyte systems; in pure LiClO4, a peak is located at 1630 cm-1 is related to vibration mode has been shifted to 1645 cm-1, 1660 cm-1, 1641 cm-1, and 1643 cm-1 with peak broadening and decrease in the intensity in all the polymer matrices. This can be understood by the miscibility of the salt with the polymer matrix has properly taken place.
Figure 4: FT-IR Spectra of PMMA(80,70,60,50):PC(10,20,30,40):PVP(10):LiClO4(5) polymer solid electrolyte system.
A peak associated with PMMA corresponding to C=O stretching with wave no. 1737 cm-1 shifts from its original position in the PPSE1, PPSE2, PPSE3 and PPSE4 at 1734 cm-1, 1726 cm-1, 1732 cm-1, 1730 cm-1 respectively. The C=O stretching at 1698 cm-1 is present in pure PC has been shifted to 1789 cm-1 in PPSE1, 1805 cm-1 in PPSE2, 1815 cm-1 in PPSE3 and 1817 cm-1 in SPE4. The peak corresponding to 3441 in PPSE1 shifts to 3448 cm-1 with peak boarding. In pure PVP a peak at 1348 cm-1 related to C-H stretching shifts to 1440 cm-1, 1448 cm-1, 1450 cm-1 and 1451 cm-1 in respective polymer electrolytes. Overall results conclude that the good complexation has been taken place in the polymer matrix 12,6.
Conclusions
FTIR spectra of solid Polymer electrolytes of PMMA(80,70,60,50): PC(10,20,30,40): PVP(10): LiClO4(5) confirms the good miscibility of all the constituent chemicals with the shifting of position in each solid electrolyte. Cole-Cole plots show the decrease in the value of Rb with an increase in temperature could be due to the existence of more number of free volumes present around the polymer chain and fast Li+ ion mobility in the polymer network favored by PC. Electric modulus studies confirm the electrolyte is an ionic conductor. Frequency-dependent Electric Modulus spectra at different temperatures show the value of M΄ is ‘0’ at lower frequencies and this could be due to the large value of capacitance associated with the electrode-electrolyte interface. The values of M΄ increases with respect to frequency this can be explained due to the short-range mobility of charge carriers and M΄΄ (imaginary) spectra explains the conduction and relaxation of conducting ions.
Acknowledgement
Authors also thank the Head Dept. of Physics for providing experiment facility.
Funding Source
Authors acknowledge DST PURSE for the financial assistance in carrying out this work.
Conflict of Interest
The authors do not have any conflict of interest.
References
- National Options for a Sustainable Nuclear Energy Systems: MCDM Evaluation Using an Improved Integrated Weighting Approach. Energies (a) Gao, R; Nam, H.O.; Ko, W.I.; Jang, H. 2017,10,2017.
CrossRef
- [2]. J. Polymer electrolytes for Lithium polymer batteries. Polym. Sci. Technol.Kang, Y.K,; Lee, C. 2003,14 396-406.
- [3]. E. Review: Characterization and modeling of the Mechanical Properties of Lithium-Ion Batteries. Energies Kermani, G.Sahraei, 2017,10,1730.
CrossRef
- [4] Ye Sol Lim, Hyun-Ah Jung and Haejin Hwang, Fabrication of PEO-PMMA-LiClO4– Based solid Polymer Electrolytes Containing Silica Aerogel Particles for All Solid-State Lithium Batteries, MDPI Energies, 11(2018), 1-10.
CrossRef
- [5] S Rajendran and T Uma, Characterization of plasticized PMMA-LiBF4 based solid polymer electrolytes, Bull. Mater Sci., 23(2000), 27-29.
CrossRef
- [6] Abdelhameed Ahmed EIBellihi, Wafaa Abdallah Bayoumy Masoud and Mahmoud Ahmed Mousa, Preparation, Characterizations and Conductivity of Composite Polymer Electrolytes Based on PEO-LiClO4 and Nano ZnO Filler, Polymer Nano Composite Electrolytes, Bull Korean Chem. Soc., 33(2012), 2949-2954.
CrossRef
- [7] Mohd Rafie Johan, Oon Hooi Shy, Suriani Ibrahim, Siti Mariah Mohd Yassin, Tay Yin Hui, Effects of Al2O3 nanofiller and EC plasticizer on the ionic conductivity enhancement of solid PEO-LiCF3SO3 solid polymer electrolyte, Elsevier,Solid State Ionics 196 (2011) 41-47.
CrossRef
- [8] M.Y.A.Rahman, A.Ahmad, T.K.Lee, Y.Farina, H.M.Dhlan, effect of Ethylene Carbonate (EC) Plasticizer on Poly (Vinyl Chloride)-Liquid 50% Epoxidised Natural Rubber (LENR50) Based Polymer Electrolyte, Scientific Research, Materials Sciences and Application, 2(2011), 818-826.
CrossRef
- [9] Hslen-Wei Chen, Tzu-Pin Lin, Fing-Chih Chang, Ionic conductivity enhancement of the plasticized PMMA/LiClO4 polymer nanocomposite electrolyte containing clay, Elsevier, Polymer 43(2002) 5281-5888.
CrossRef
- [10] Rajani Sharma, Anjan Sil, Subrata Ray, Characterization of plasticized PMMA-LiClO4 solid polymer electrolytes, Advanced Materials Research, 585(2012), 185-189.
CrossRef
- [11] P. Pal and A. Ghosh, Charge carrier dynamics in PMMA-LiClO4 based polymer electrolytes plasticized with different plasticizers, Journal of Applied Physics, 122(2017).
CrossRef
- [12] Characterization and ion transport studies through impedance spectroscopy on (1-x)Pb(NO3)2:xAl2O3 composite solid electrolytes, Y Govinda Reddy, AM. Awasthi, A.Sadananda Chary & S.Narender Reddy, Emergent Materials,1(2018), 175–184.
CrossRef
- [13] Gh. Mohammed, Adel M.EI sayed, S. EI. Gamal, Effect of M Nitrates on the Optical, Dielectric Relaxation and Porosity of PVC/PMMA Membranes(M=Cd, Co, Cr or Mg), Journal of inorganic & organometallic polymers & materials, springer science + business media, Lc part of springer nature, 2019, 14pages.
CrossRef
- [14] Nor Hazlizaaini Basri & N.S.Mohammed, conductivity studies and dielectric behavior of pvdf-hfp-pvc-liclo4 solid polymer electrolyte, Solid State Science & Technology, 17(2009), 63-72.
Views: 635
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , A. Sadananda Chary2, Venkata Ramana Jeedi 3, Yalla Mallaiah 4 and S. Narender Reddy5
, A. Sadananda Chary2, Venkata Ramana Jeedi 3, Yalla Mallaiah 4 and S. Narender Reddy5 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal