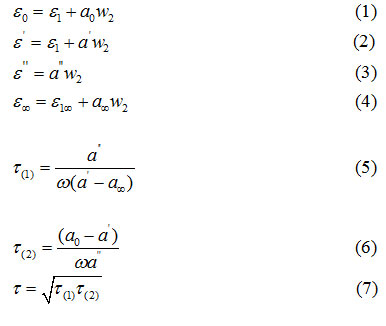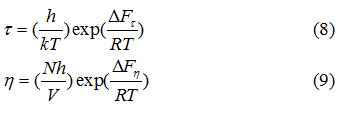Dielectric Relaxation Studies Between Brompheniramine with 1-Butanol, 1-Pentanol and 1-Hexanol at 303K
Sampandam Elangovan* , Tilahun Diriba Garbi
, Tilahun Diriba Garbi and Senbeto Kena Etana
and Senbeto Kena Etana
Department of Physics, College of Natural and Computational Science, Wollega University, Nekemte, Ethiopia-395
Corresponding Author E-mail: elangovan.physics@rediffmail.com
DOI : http://dx.doi.org/10.13005/msri/170305
Article Publishing History
Article Received on : 20-November-2020
Article Accepted on : 23-Dec-2020
Article Published : 25 Dec 2020
Plagiarism Check: Yes
Reviewed by: Dr. RAMA RAO M
Second Review by: Dr. Kriti Sahu
Final Approval by: Dr. Nagaraja K K
Article Metrics
ABSTRACT:
Dielectric parameters such as the dielectric constant (є’), dielectric loss (є’’), static dielectric constant (є0), dielectric constant at an optical frequency (є∞), dielectric relaxation time(τ), change in activation free energies (ΔFτ, ΔFη) of brompheniramine with 1-butanol, 1-pentanol and 1-hexanol are determined for the various concentrations at 303K. The dielectric relaxation time (τ) is determined by Higasi and Cole-Cole method. The static dielectric constant (є0) and relaxation time (τ) are decreased with increasing the concentration of brompheniramine in the selected 1-alcohol systems. The dielectric relaxation time (τ) increased with an increase in the chain length of the 1-alcohols. The results confirm that the intermolecular interaction depends upon the carbon chain length of the alcohols. The strength of the interaction between brompheniramine with 1- alcohols is 1-butanol < 1-pentanol <1-hexanol.
KEYWORDS:
Brompheniramine; Dielectric Relaxation; 1-Butanol; 1-Hexanol; 1-Pentanol
Copy the following to cite this article:
Elangovan S, Garbi T. D, Etana S. K. Dielectric Relaxation Studies Between Brompheniramine with 1-Butanol, 1-Pentanol and 1-Hexanol at 303K. Mat. Sci. Res. India; 17(3).
|
Copy the following to cite this URL:
Elangovan S, Garbi T. D, Etana S. K. Dielectric Relaxation Studies Between Brompheniramine with 1-Butanol, 1-Pentanol and 1-Hexanol at 303K. Mat. Sci. Res. India; 17(3). Available from: https://bit.ly/3ruQLdh
|
Introduction
The dielectric relaxation studies are vital in analyzing the strength of the inter molecular interaction between the binary liquid systems [1-4]. Jyostna et al. [5] reported thermodynamic parameters of isoamyl alcohols and mono clinic aromatic liquid mixtures. Shakila et al. [6] studied the dielectric properties of aromatic alcohols and aliphatic amines at different temperatures. In general, dielectric relaxation time varies with the inter molecular forces acting between the molecules in the selected liquid mixtures. Brompheniramine is one of the critical compounds of an amine group with spectacular applications, including pharmaceutical industries [7]. Higher carbon chain length alcohols are having self associated and proton donating ability in the liquid mixtures. The variations in the dielectric constant (є’), dielectric loss (є’’), static dielectric constant (є0) and the dielectric constant at an optical frequency (є∞) with a range of brompheniramine concentrations with 1-butanol,1-pentanol and 1-hexanol systems are useful in the applied research and chemical industries. Moreover, the variations in the dielectric constant and dielectric relaxation time should be useful in the analysis of intermolecular interaction between the functional group of the selected liquid mixtures. This research work attempts to analyse the intermolecular interaction between the brompheniramine and 1-butanol,1-pentanol and 1-hexanol at 303K using time domain reflectometry techniques.
Materials and Methods
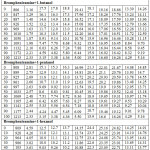
Brompheniramine and alcohols (Purity 99%, AR grade) were procured from E-Merck India. Furthermore, these chemicals were purified by adopting the double distillation method under reduced pressure [8]. Airtight sample bottles were used to keep the samples, to avoid any moisture . In order to validate the purity of the samples, density and viscosity were determined and compared with the available literature [9,10] as listed in Table 1. The dielectric constant (ε’) and dielectric loss (ε’’) have been measured using an X-band microwave frequency oscillator of frequency 9.36 GHz at 303K. Ostwald’s viscometer was used to determine the viscosity of the liquid mixture. The specific gravity bottle (5cc) was used to measure the density of the liquid system.
Theory
Higasi’s method [11] was used to determine the dielectric relaxation time (τ) of the liquid mixtures. Here, the static dielectric constant (є0), dielectric constant (є’), dielectric loss (є’’), and the dielectric constant at high frequency 1015Hz were determined by using the following relations. The slopes a0, a’, a’’ and a∞ were calculated from the observed data.
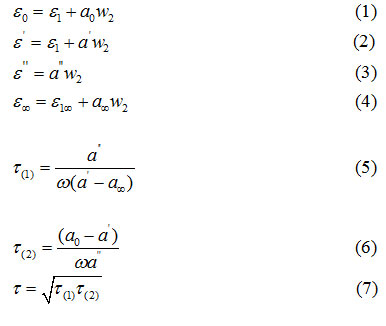
Here (τ) is the mean dielectric relaxation time. Eyring’s equation [12] was used to calculate dielectric relaxation ΔF τ and viscous flow ΔF η
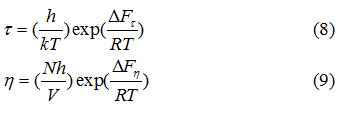
Where h is Planck’s constant, k is Boltzmann constant, N is Avogadro number and V is the molar volume. The measured values of ε0, ε,’ ε’’ and ε∞ are fitted in a complex plane plot with depressing circular arc.
(ωτ)1-α = V/U (10)
Where ω is the angular frequency and α can be determined by using Cole-Cole plot.
Results and Discussion
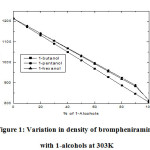
Various physicochemical parameters of brompheniramine with 1-butanol,1-pentanol,1-hexanol are determined at 303K and listed in Table 1. The density of the liquid mixture increases with increasing the concentrations of brompheniramine. Furthermore, density increased with the carbon chain length of the alcohols due to the formation of more compact dipoles in the given volume of the liquid system. The same trend is observed in the viscosity of the solution as listed in Table 1. The increasing trend of density with increasing carbon chain length of alcohols in brompheniramine medium is as shown in Figure 1. It may due to the dispersive forces acting between the selected liquid system [13-15]. In general, the dielectric relaxation time is influenced by the shape and size of the rotational and vibrational characteristics of the dipoles present in the liquid mixtures [16-18].
Table 1: Physical properties of the pure liquids at T =303K
|
S.No
|
Liquid
|
Density
( ρ ) kgm-3
|
Viscosity
( η ) ×10-3 Nm-2s
|
Reference
|
|
Expt
|
Lit
|
Expt
|
Lit
|
|
1
|
Brompheniramine
|
1213
|
1212
|
2.3297
|
2.3254
|
[9]
|
|
2
|
1-butanol
|
806
|
804
|
1.162
|
1.150
|
[10]
|
|
3
|
1-pentanol
|
808
|
807
|
2.813
|
2.766
|
[10]
|
|
4
|
1-hexanol
|
809
|
810
|
3.498
|
3.513
|
[10]
|
Figure 1: Variation in density of brompheniramine with 1-alcohols at 303K
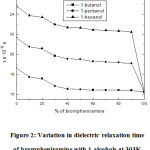
The dielectric relaxation time (τ) decreases with increasing the concentrations of brompheniramine, as shown in Figure 2. However, the dielectric relaxation time decreased upto a certain percentage (40%) of brompheniramine then almost saturated. It signifies that the 1:1 complex formation in the binary system. Further, the dielectric parameters (ε0, ε,’ ε’’ and ε∞) are decreased with increasing the brompheniramine concentrations as listed in Table 2. This trend suggested that the formation of more dipoles in the liquid mixtures. This result is in accordance with the methylacrylate with 1-alcohol system, which has been reported by Dharmalingam et al. [19].
Table 2: Various parameters of brompheniramine with alcohols at 303K
Figure 2: Variation in dielectric relaxation time of brompheniramine with 1-alcohols at 303K
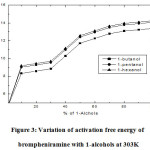
The increasing activation free energy with the concentrations of 1-alcohols is as shown in Figure 3. It signifies that the activation free energy increased exponentially in the 1-alcohol rich concentrations.. The corresponding variations suggested the increasing dipoles in the self associative nature of the 1-alcohols. Moreover, the increasing carbon atoms in the mixture provides more dipoles, hence the activation free energy is greater in the 1-hexanol as compare with the 1-butanol and 1-pentanol. The free energy activation due to dielectric relaxation (ΔFτ) is less than that of the molar free energy of activation for viscous flow (ΔFη) is listed in Table 3. It may expose the viscous flow influenced by both the molecules’ vibrational and rotational motion in the liquid mixture [20]. The results confirm that the occurrence of N-H….O-H bonding between the functional groups present in the system. The systematic observation puts forwards the influence of the proton donating abilities of the alcohols increasing with their concentrations and carbon chain length in the brompheniramine medium. Hence the strength of inter molecular interaction of 1-alcohols with brompheniramine is observed in the order of 1-butanol<1-pentanol<1-hexanol.
Figure 3: Variation of activation free energy of brompheniramine with 1-alcohols at 303K
Conclusion
Several physicochemical properties of brompheniramine and 1-butanol, 1-pentanol and 1-hexanol are analysed at 303K. The significant changes in the dielectric relaxation time suggested that the existence of intermolecular interaction between the amine and hydroxyl group present in the systems. The considerable variations in the various parameters suggested that the intermolecular interaction between brompheniramine and 1-alcohols is in the order of 1-butanol<1-pentanol<1-hexanol. This result leads to analyse the further interactions of the brompheniramine with 2-alcohols and alkoxy alcholols for the benefit of the applied research to study the intermolecular intermolecular interactions among the liquid mixtures
Acknowledgment
The authors are thankful to the Research and Technology Transfer Centre, Wollega University, Nekemte, Ethiopia for providing the necessary facilities to complete this work.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding Source
The authors declare that the funding is done by the research project fund (WUS1-108) sanctioned by Research and Technology Transfer Centre, Wollega University, Nekemte, Ethiopia.
References
- Y. Liu, Yg. Liu, Q. Wang, Y.Han and Y. Tan, Boronic ester-based self-healing hydrogels formed by using intermolecular B-N coordination. Polymer. 202, 122624 (2020). https://doi.org/10.1016/j.polymer.2020.122624.
CrossRef
- S. Mora, R. Tayouo, B. Boutevin, G. David and S. Caillol, A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 123, 109460 (2020) . ttps://doi.org/10.1016/j.eurpolymj.2019.109460.
CrossRef
- T. Wang, H.B.Xie, Z. Song, J. Niu, De-Li Chen, D. Xia, J. Chen, Role of hydrogen bond capacity of solvents in reactions of amines with CO2: a computational study. J. Environ. Sci. 91, 271-278 (2020).
CrossRef
- D.Zhao, Y. Zhuang, C. Fan, F. Yang, Y. Chen and X. Zhang, Surface tension of binary mixtures composed of N, N-dimethylcyclohexylamine and alcohols at different temperatures.J.Chem.Thermodyn.143,106041(2020). https://doi.org/10.1016/j.jct.2019.106041
CrossRef
- T. Savitha Jyostna, B. Satheesh, D. Sreenu, G. Ramesh and E. Jayanthi Rani, The study of thermo-physical properties of binary liquid mixtures of isoamyl alcohol with amines at 298.15–308.15K. Phys. Chem. Liq, 58, 349-363 (2020).
CrossRef
- Shakila, S. Ravikumar, V. Pandiyan and R. Gaba, Thermodynamic properties of binary liquid mixtures containing aromatic alcohol and aliphatic amines at different temperatures. J. Mol. Liq. 2851, 279-287 (2019).
CrossRef
- S.Elangovan, K. Assefa and Y. A. Abbo, Intermolecular Interactions of Brompheniramine with 1-Pentanol at Various Temperatures. Elixir Ultrasonics 115, 49704-49707(2018).
- D.D.Perrin and W.L.F. Armarego Purification of Lab Chem, 3rd Edn, (Pergamon Press, Oxford), (1980).
- E. Sampandam, T. Diriba Garbi and Y. Alemu Abbo, Intermolecular interaction studies of brompheniramine with 1-propanol at 303 K, 308 K and 313 K. Chemistry Africa (2020). https://doi.org/10.1007/s42250-020-00155-2.
CrossRef
- S.Thirumaran and E. Jayakumar, Ultrasonic study of n-alkanols in toluene with nitrobenzene. Indian J. Pure. Appl Phys. 47, 265-272 (2009).
- K.Higasi, Y. Koga, M. Nakamura, Dielectric relaxation and molecular structure. V. application of the single frequency method to systems with two Debye dispersions. Bull. Chem Soc. Japan. 44, 988-992 (1971).
CrossRef
- H. Eyring, Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J. Chem. Phys, 4, 283-294 (1926).
CrossRef
- S. Pradhan and S.Mishra, An eye on molecular interaction studies of non-aqueous binary liquid mixtures with reference to dielectric, refractive properties and spectral characteristics. J. Mol. Liq. 279, 317-326 (2019).
CrossRef
- Y.Monascal, L. Cartaya, A. A. Aular, A. Maldonado and G.Chuchani, The ion pair mechanism in the thermal deamination of primary amines catalyzed by HBr in the gas phase: DFT and AIM analysis. Chem. Phys. Lett.703, 117-123 (2018).
CrossRef
- X.Li, J. Ke, J. Wang, C.Liang, M. Kang, Y. Zhao and Q. Li, A new amino-alcohol originated from carbon dioxide and its application as chain extender in the preparation of polyurethane.J. Co2. Util. 26, 52-59 (2018).
CrossRef
- M.Raveendra, M.Chandrasekhar, , A.Venkatesulu, K.Sivakumar and K. Reddy, Study on thermo physical properties of binary mixture containing aromatic alcohol with aromatic, substituted aromatic amines at different temperatures interms of FT-IR, 1H NMR spectroscopic and DFT method. Fluid Phase Equilib. 462, 85-99 (2018).
CrossRef
- T. Vishwam, N.K.S.P.S.Sarma, V.R.K.Murthy and S.S.Sastry. Dielectric relaxation studies of acetonitrile/propylene glycol and their binary mixtures. Indian J. Pure Appl. Phys.55, 403-412 (2017).
- J.G. Kirkwood, The Dielectric Polarization of Polar Liquids. J. Chem Phys, 7, 911-919 (1939).
CrossRef
- K. Dharmalingam, K. Ramachandran, P. Sivagurunathan, B. Prabhakar Undre, P. W. Khirade, and S. C. Mehrotra. Dielectric Study of methyl acrylate-alcohol mixtures using Time Domain Reflectometry. Bull. Korean Chem. Soc.27, 2040-2044 (2006).
CrossRef
- S. Elangovan and D.W. Amente, Intermolecular interaction of methyl formate with 1-butanol, 1-pentanol and 1-hexanol at 303K. Material. Sci. Res. India.14, 212-214 (2017).
CrossRef
Views: 421
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Tilahun Diriba Garbi
, Tilahun Diriba Garbi and Senbeto Kena Etana
and Senbeto Kena Etana
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal