Synthesis, Spectroscopic Characterization, Quantum Chemical Study and Antimicrobial Study of (2e) -3-(2, 6-Dichlorophenyl) -1-(4-Fluoro) -Prop-2-En-1-One
Nutan V.Sadgir1 *, Sunil L.Dhonnar1, Bapu Jagdale2, Bhagyashri Waghmare3 and Chetan Sadgir4
*, Sunil L.Dhonnar1, Bapu Jagdale2, Bhagyashri Waghmare3 and Chetan Sadgir4
1Department of Chemistry, L.V.H. Arts, Science, and Commerce College, Panchavati, Nashik (M.S) India.
2Department of Chemistry, Arts, Science, and Commerce College, Manmad, Nashik (M.S) India.
3Department of Chemistry M.S.G. Arts, Science and Commerce College,Malegaon,Nashik(M.S.) India
4Zydus Cadila Ahmedabad,Gujrat,India
Corresponding Author Email: nutansadgir@gmail.com
DOI : http://dx.doi.org/10.13005/msri/170311
Article Publishing History
Article Received on : 10-September-2020
Article Accepted on : 19-Nov-2020
Article Published : 23 Nov 2020
Plagiarism Check: Yes
Reviewed by: Sapana Jadoun
Second Review by: Rizwan Arif
Final Approval by: Ramachandra Naik
Article Metrics
ABSTRACT:
In the present work (2E) -3-(2, 6-dichlorophenyl) -1-(4-Fluoro) -prop-2-en-1-one was Prepared by Claisen-Schmidt condensation .Synthesized molecules were characterized by using FTIR, 1H NMR spectroscopy.Molecular geometry,Vibrational frequency of title compound were calculated using the DFT/B3LYP method with 6-311++G (d, p) basis set.The experimentally obtained FTIR spectra were in good agreement with calculated infrared spectrum.The FMO and molecular electrostatic potentialwere performed to study the reactivity of molecules at the same levelof theory. The synthesized compound shows moderate antimicrobial activity
KEYWORDS:
Chalcone; DFT; FMO; Gaussian-03; UV-Visible
Copy the following to cite this article:
Sadgir N. V, Dhonnar S. L, Jagdaleb B, Waghmare B, Sadgird C, Synthesis, Spectroscopic characterization, Quantum Chemical studyand antimicrobial study of (2E) -3-(2, 6-dichlorophenyl) -1-(4-Fluoro) -prop-2-en-1-one.Mat. Sci. Res. India; 17(3).
|
Copy the following to cite this URL:
Sadgir N. V, Dhonnar S. L, Jagdaleb B, Waghmare B, Sadgird C, Synthesis, Spectroscopic characterization, Quantum Chemical studyand antimicrobial study of (2E) -3-(2, 6-dichlorophenyl) -1-(4-Fluoro) -prop-2-en-1-one.Mat. Sci. Res. India; 17(3). Available from: https://bit.ly/3pVfLcD
|
Introduction
The Chalcone is a simple scaffold found in many naturally occurring compounds mainly in flavonoids and isoflavonoids in plants or can be synthesized in laboratory by different methods. Basically chalcones are alpha, beta unsaturated ketone, in which two aromatic rings joined at 1 and 3 position (1,3-diphenyl-2E-propene-2-one).The two rings of chalcones are interconnected by electrophilic nature of alpha beta unsaturated carbonyl system, which are having complete delocalisation on both aromatic rings. It exists as cis and trans isomer in which trans isomer is thermodynamically more stable. They display a wide range of pharmacological activities like antimicrobial[4,5], antifungal [6] anticancer [7],antileishmanial [8],anti-HIV [9], anti-inflammatory [10], anti-tuberculosis [11] ,anticonvulsant [12] , ant-viral [13],anti-oxidant [14], anti-diabetic [15] etc.Chalcone act as a unique template for synthesis of different heterocyclic like Pyrazoline, Oxazolines, pyrimidinesetc.
Density Functional Theory has been very popular in describing the structural and electronic properties of atoms and molecules. The main objective of this paper to synthesized title compound and study their experimental and computational investigation on the Molecular structure, vibrational spectra, and electronic properties. We have synthesized of 2E)-3-(2, 6-dichlorophenyl)-1-(4-Fluoro)-prop-2-en-1-one by Claisen-Schmidt condensation and study their molecular structure, and Vibrational Frequencies investigation by DFT at B3LYP/6-311++G(d,p) level, In addition to this FMOs, ionization potential, electronegativity, global Electrophilicity index, chemical potential were studied by using a 6-311++G (d, p) basis set.
Experimental
Material and Physical Measurement
The Chemicals used for synthesis are of AR grade.Melting point of the compound was determinedin an open capillaries and uncorrected.FTIR spectrum of title compound was recorded on Shimadzu spectrometer using KBr pellets. The 1H NMR was recordedon Brucker Avance 500MHZ spectrometer using TMS as an internal standard. Reaction is monitored by thin layer chromatography by using n-hexane and ethyl acetate solvent system.
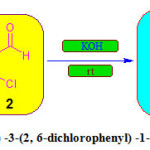
Synthesis of (2E) -3-(2, 6-dichlorophenyl) -1-(4-4-Fluoro) -prop-2-en-1-one
The 4-Fluoroacetophenone (0.01 mole) and 2, 6dichloro benzaldehyde (0.01 mole) were dissolved in ethyl alcohol and 5ml of KOH (20%) was added drop wise to the solution with stirring at room temperature. The completion of the reaction was monitored by TLC. After completion of reaction, reaction mixture poured into crushed ice and acidified with dil. HCl.The solid compound obtained was filtered, driedand recrystallized from ethyl alcohol.
Yield: 87%, FT-IR (KBr,cm-1): 3076(aromatic C-H), 2839(C-H), 1664 (C=O), 1602 (C=C),1508 (aromatic C=C), ; 1H NMR (500 MHz,CDCl3,δ/ppm): 8.05 (d, J= 8.5 Hz.2H), 7.84 (d,J = 16 Hz,1H), 7.63(d, J =16 Hz,1H), 7.387 (d,J =8 Hz,2H), 7.170-7.237 (m,3H).
Scheme 1: Synthesis of (2E) -3-(2, 6-dichlorophenyl) -1-(4-4-Fluoro) -prop-2-en-1-one
Computational Details
The Density functional theory (DFT) calculations were performed on an Intel (R) Core (TM) i7 personal computer using Gaussian -03 program package[17]. Thegeometry of the title compound was optimized by DFT /B3LYF method with 6-311++G (d, p) basis set level [18-20]. To explore the electronic properties, the theoretical UV-Visible spectra have been investigated by the TD-DFT method with a 6-311++G (d, p) basis in the gas phase.
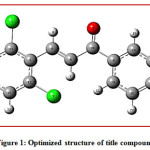
Figure 1: Optimized structure of title compound
Results and Discussion
Molecular Geometry
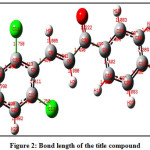
The optimize structure of the title compound with the labelling of atoms shown areFig.1 The molecular structure of the title compound contains two six membered rings in which, one is 2, 6-dichloro substituted ring attach to C=C of enone system (ring B) and another ring is a 4-Fluoro substituted ring attach to carbonyl group (ring A).The α, β-unsaturated ketone is confirmedby the shorter bond length O2-C20 and C6-C8 are found to be 1.223Å, 1.34Årespectively. The C 13-F28, Cl1-C23 and Cl2-C12 bond lengths are 1.352 Å, 1.757Å and 1.759Å respectively and foundwithin the normal range.Allthe bond lengths are within the normal range and compared with the previously reported structure [22-26].The calculated C 20-C6,C8-C5torsion angle is 179.50 confirms the molecule exhibits E configuration, which shows trans geometry.
Figure 2: Bond length of the title compound
Table 1: The bond length and dihedral angle of title compound calculated at B3LYP/6-311++G (d, p) level
|
Bond
|
Bond Length
[Å]
|
Bond
|
Bond Angle
[°]
|
|
Cl(1)-C(23)
|
1.757
|
Cl(1)-C(23)-C(5)
|
119.64
|
|
Cl(2)-C(12)
|
1.759
|
Cl(2)-C(12)-C(5)
|
121.39
|
|
O(3)-C(20)
|
1.223
|
O(3)-C(20)-C(4)
|
120.02
|
|
C(4)-C(20)
|
1.499
|
O(3)-C(20)-C(6)
|
120.8
|
|
C(4)-C(14)
|
1.404
|
C(4)-C(10)-C(26)
|
120.97
|
|
C(4)-C(10)
|
1.402
|
C(20)-C(4)-C(10)
|
123.9
|
|
C(5)-C(8)
|
1.470
|
H(9)-C(8)-C(6)
|
116.54
|
|
C(5)-C(23)
|
1.412
|
H(7)-C(6)-C(8)
|
120.99
|
|
C(5)-C(12)
|
1.411
|
H(7)-C(6)-C(20)
|
119.24
|
|
C(6)-C(20)
|
1.490
|
C(5)-C(8)-H(9)
|
115.86
|
|
C(6)-C(8)
|
1.340
|
C(5)-C(8)-C(6)
|
127.54
|
|
C(6)-H(7)
|
1.080
|
C(8)-C(6)-C(20)
|
119.76
|
|
C(8)-H(9)
|
1.086
|
C(20)-C(6)-H(7)
|
119.24
|
|
C(10)-C(26)
|
1.392
|
C(10)-C(4)-H(14)
|
118.59
|
|
C(10-H(11)
|
1.080
|
H(15)-C(14)-C(4)
|
118.21
|
|
C(12)-C(21)
|
1.391
|
H(15)-C(14)-C(16)
|
120.60
|
|
C(13)-C(16)
|
1.389
|
C(4)-C(14)-C(16)
|
121.19
|
|
C(13)-C(26)
|
1.385
|
C(14)-C(16)-H(17)
|
121.82
|
|
C(13)-F(28)
|
1.352
|
C(14)-C(16)-C(13)
|
118.33
|
|
C(14)-H(15)
|
1.083
|
C(16)-C(13)-F(28)
|
118.77
|
|
C(14)-H(16)
|
1.387
|
C(13)-C(16)-H(17)
|
119.84
|
|
C(16)-H(17)
|
1.083
|
F(28)-C(13)-C(26)
|
118.74
|
|
C(18)-H(19)
|
1.083
|
H(27)-C(26)-C(13)
|
119.90
|
|
C(18)-C(21)
|
1.390
|
H(27)-C(26)-C(10)
|
121.67
|
|
C(18)-C(24)
|
1.390
|
H(11)-C(10)-C(26)
|
117.99
|
|
C(21)-H(22)
|
1.082
|
C(5)-C(12)-C(21)
|
122.46
|
|
C(23)-C(24)
|
1.388
|
H(22)-C(21)-C(12)
|
119.38
|
|
C(24)-H(25)
|
1.082
|
H(22)-C(21)-C(18)
|
120.85
|
|
C(26)-H(27)
|
1.083
|
H(19)-C(18)-C(21)
|
119.91
|
|
|
|
H(19)-C(18)-C(24)
|
119.99
|
|
|
|
C(18)-C(24)-H(25)
|
121.12
|
|
|
|
C(23)-C(24)-H(25)
|
119.69
|
Figure 3: a) The Graphical representation of bond length and b) Bond Angle
Vibrational frequencyAssignment
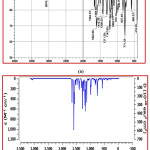
Comparison of vibrational assignment of title compound by Experimental IR spectrum with theoretical IR spectrum. Experimental IR spectrum is recorded in the region of 4000-500 cm–1(Solid phase) and calculated vibrational spectrum in the gas phase. Title compound contains total 28 atoms with 78 fundamental modes of vibration. Theoretical and experimental IR spectrum is shown in Fig 4a and 4b.Carbonyl stretching frequency of alpha beta unsaturated ketone in the range of 1700-1600 cm-1, the experimental carbonyl stretching frequency of title compound 1658 cm-1 and theoretically it is 1664 cm-1 this confirms presence of carbonyl group, lower value of IR frequency is due to conjugation of enone system with the aromatic rings. Experimental IR at 1525cm-1 and theoretical IR at 1508cm-1 is due to the Ar-C=C- stretching vibrations. Outof plane bending vibrations experimentally at 972.12 cm-1 and theoretically at 972cm-1shows trans geometry of alkene. The C-H experimental and theoretical is stretching vibration 3076 cm-1 and 3072cm-1respectively.
Figure 4: a) Experimental FT-IR spectrum and b) Simulated IR spectrum of title compound
Table 2: Selected Experimental and theoretical vibrational assignments of title compound.
|
Selected mode no
|
Calculated (Scaled) frequencies in cm-1
|
Calculated IR intensity
|
Experimental Frequencies cm-1
|
Assignment
|
|
78
|
3104
|
4.25
|
–
|
υsym (10)C-(11)H ring b+ υsym (6)C-(7)H(C=C)
|
|
77
|
3087
|
2.29
|
–
|
υsymCH (Ring a)
|
|
76
|
3085
|
5.50
|
–
|
υsym CH (Ring b)
|
|
75
|
3083
|
0.86
|
–
|
υsym CH (Ring b)
|
|
74
|
3082
|
0.24
|
–
|
υasymm (Ring a)
|
|
73
|
3072
|
0.189
|
3076
|
υasymm (Ring b) CH+ υsym (6)C-(7)H(C=C)
|
|
72
|
3070
|
5.17
|
–
|
υasymm (Ring b)
|
|
71
|
3061
|
2.64
|
–
|
υasymm (Ring a)
|
|
70
|
3042
|
1.69
|
–
|
(8)C- (9)H(C=C)
|
|
69
|
1656
|
136.31
|
1664.57
|
υ C=O
|
|
68
|
1590
|
289.29
|
1602.85
|
υ C=C,C=O
|
|
67
|
1572
|
129.83
|
–
|
υ C=C and CH (Ring b)
|
|
66
|
1560
|
19.88
|
–
|
υ C=C and CH (Ring b)
|
|
65
|
1554
|
18.02
|
–
|
υ C=C and CH (Ring a)
|
|
64
|
1525
|
33.85
|
1508
|
υ C=C and CH (Ring a)
|
|
63
|
1475
|
35.93
|
1498.69
|
υ C=C and CH (Ring b)
|
|
62
|
1405
|
42.57
|
1415.75
|
υ C=C and CH (Ring a)
|
|
60
|
1381
|
20.89
|
–
|
υ C=C and CH (Ring b)
|
|
59
|
1312
|
49.65
|
1300.02
|
υ C=C
|
|
58
|
1289
|
17.59
|
–
|
υ C=C and CH (Ring b)
|
|
55
|
1242
|
67.61
|
–
|
υ C=C (Ring a)
|
|
46
|
1048
|
2.56
|
1093.64
|
υ C=C and CH (Ring a)
|
|
45
|
992
|
53.00
|
1008.77
|
υ C=C and CH (Ring b)
|
|
43
|
972
|
39.33
|
972.12
|
υ γCH (C=C)
|
|
40
|
921
|
1.54
|
900.76
|
υ CH (Ring a)
|
|
39
|
879
|
8.54
|
–
|
υ CH(C=C)
|
|
36
|
827
|
36.16
|
827.46
|
υ CH (Ring a)
|
|
35
|
810
|
23.98
|
–
|
υ C-(28)F
|
|
34
|
794
|
1.83
|
–
|
υCH (Ring b)
|
|
33
|
760
|
3.36
|
771.53
|
Υ CH (Ring b)
|
|
31
|
748
|
91.73
|
–
|
υ C-(1)Cl
|
|
v- stretching; asym-asymmetric; sym-symmetric; def-deformation; β-In-plane bending; γ-out of plane bending, ρ-rocking, Г-torsion
|
UV-Vis Spectra Study and Global Chemical Reactivity Parameters
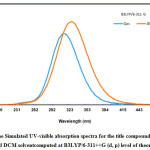
The absorption energies (λ in nm), oscillator strength (ƒ), and electronic transitions of the title compound have been computed at the TD-DFT B3LYP/6-311++G (d, p) level of theory for optimized geometry. To study the effect of solvent on the wavelength of absorption, TD-DFT calculation, perform in both gas and DCM solvent. The calculated electronic transitions of high oscillatory strength, wavelength are given in Table 3. The first singlet state (S1) is found to be at 366 nm in DCM (Fig.5)and 378nm in the gas phase (Fig.5).The second singlet excited state (S2) is present at 326 nm (DCM) and 314 nm (gas phase).The third singlet excited state (S3) is present at 322 nm (DCM) and 311 nm (gas phase).The Simulated spectrum (Gas and DCM) is illustrated in Fig.5The assigned bands were characterized by (n→π*) and (π→π*) transitions.
Table 3: Absorption energies (λ in nm), oscillator strength (ƒ), and transitions of title compound computed at TD-DFT B3LYP/6-311++G (d, p) level of theory
|
State
|
DCM
|
Gas Phase
|
|
Config
|
f
|
λ, nm
|
Excitation energy (eV)
|
Config
|
f
|
λ, nm
|
Excitation energy(eV)
|
|
I
|
72 -> 76
73 -> 76
75 -> 76
|
0.0234
|
366
|
3.3863
|
72 -> 76
74 -> 76
75 -> 76
|
0.0066
|
378
|
3.2723
|
|
II
|
72 -> 76
|
0.6083
|
326
|
3.7921
|
74 -> 76
|
0.5315
|
314
|
3.9478
|
|
|
73 -> 76
|
|
|
|
75 -> 76
|
|
|
|
|
|
74 -> 76
|
|
|
|
–
|
|
|
|
|
|
75 -> 76
|
|
|
|
–
|
|
|
|
|
III
|
74 -> 76
|
0.0703
|
322
|
3.8491
|
72 -> 76
|
0.0560
|
311
|
3.9791
|
|
|
75 -> 76
|
|
|
|
73 -> 76
|
|
|
|
|
|
75 -> 78
|
|
|
|
74 -> 76
|
|
|
|
|
|
–
|
|
|
|
75 -> 76
|
|
|
|
|
|
–
|
|
|
75 -> 78
|
|
|
|
|
IV
|
71 -> 76
|
0.0523
|
294 4.2073
|
71 -> 76
|
0.0272
|
291
|
4.2563
|
|
|
72 -> 76
|
|
|
72 -> 76
|
|
|
|
|
|
73 -> 76
|
|
|
73 -> 76
|
|
|
|
|
|
75 -> 76
|
|
|
74 -> 76
|
|
|
|
|
|
–
|
|
|
75 -> 76
|
|
|
|
|
V
|
71 -> 76
|
0.0373
|
286 4.3229
|
71 -> 76
|
0.0165
|
281
|
4.4066
|
|
|
72 -> 76
|
|
|
72 -> 76
|
|
|
|
|
|
73 -> 76
|
|
|
74 -> 79
|
|
|
|
|
|
73 -> 79
|
|
|
–
|
|
|
|
Figure 5: The Simulated UV-visible absorption spectra for the title compound in vacuum and DCM solventcomputed at B3LYP/6-311++G (d, p) level of theory.
Global Chemical Reactivity Descriptors
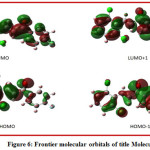
The highest molecular orbital and lowest molecular orbital are called frontier molecular orbitals. The HOMO-LUMO energy value and energy gap values for title compound were computed by the TD-DFT method at B3LYP/6-311G (d, p) basis set in gas phase.HOMO-LUMO plot of title compound is shown in Fig.6the computed gas phase HOMO and LUMO energies are -7.2328 eV and -2.7225 eV respectively.Whereas the energy gap of title compound is4.5103eV. From the HOMO-LUMO energies various chemical reactivity parameters have been derived. The Ionization potential (I), Electron Affinity (A), Chemical hardness (ɳ), Chemical softness (S), Electronic chemical potential (μ), Global Electrophilicity index (ω) parameters were calculatedbased on Koopman’s theorem [26]equation 1-4 [27-30]. The HOMO-LUMO energies and global reactivity parameters are listed in table 5.The global hardness (η ) of 2.2551eV, Chemical Softness (S ) 0.4434eV, chemical potential ( μ) -4.9777 eV, electrophilicity Index ( ω) 4.8554eV suggest the good stability of the compound.
ɳ = ½ (I – A)…… (1)
S = 1/η….. (2)
μ = -½ (I+A)… (3)
ω = μ2 / 2η ….. (4)
Figure 6: Frontier molecular orbitals of title Molecule
Table 4: Global chemical reactivity indices calculated at B3LYP/6-311G++(d,p) level
|
Parameters
|
B3LYP/6-311++G(d,p)
|
|
ELUMO(eV)
|
-2.7225eV
|
|
EHOMO(eV)
|
-7.2328eV
|
|
D E= ELUMO-EHOMO(eV)
|
4.5103eV
|
|
Electron affinity(A)
|
2.7225eV
|
|
Ionization Energy(I)
|
7.2328eV
|
|
Global Hardness (η)
|
2.2551eV
|
|
Chemical Softness (S)
|
0.4434eV
|
|
Electronic chemical potential (μ)
|
-4.9777eV
|
|
Global electrophilicity Index (ω)
|
4.8554eV
|
Mulliken Atomic Charges
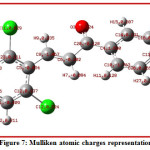
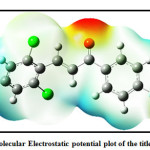
The Mulliken atomic charges play an important role to know the chemical reactivity of compounds. The Mulliken atomic charges calculated and reported of title compound is shown in Table 5.As indicated in table 5The C4 atom carries the larges positive charge 1.657 among other carbon atoms and therefore expected to be the site for nucleophilic attack in title compound, whereas C8 and C10 carries higher negative charge -1.225 and -1.633 respectively in all carbon atoms. The Molecular electrostatic potential plot is shown in Fig.8,this gives information about the chemical reactivity of sites. The dipole moment of the title compound is 3.9 Debye indicates polar nature.
Figure 7: Mulliken atomic charges representation
Table 5: Mulliken atomic charges of Title compound at B3LYP/6-311++G (d, p) level
|
Atom
|
Charge
|
Atom
|
Charge
|
|
1Cl
|
0.635
|
15 H
|
0.208
|
|
2 Cl
|
0.543
|
16 C
|
-0.008
|
|
3 O
|
-0.223
|
17 H
|
0.204
|
|
4 C
|
1.657
|
18 C
|
-0.667
|
|
5 C
|
0.333
|
19 H
|
0.170
|
|
6 C
|
-0.317
|
20 C
|
-0.222
|
|
7 H
|
-0.058
|
21 C
|
-0.151
|
|
8 C
|
-1.225
|
22 H
|
0.211
|
|
9 H
|
0.240
|
23 C
|
-0.172
|
|
10 C
|
-1.633
|
24 C
|
0.001
|
|
11 H
|
0.101
|
25 H
|
0.212
|
|
12 C
|
0.324
|
26 C
|
-0.144
|
|
13 C
|
-0.618
|
27 H
|
0.214
|
|
14 C
|
0.548
|
28 F
|
-0.164
|
Figure 8: Molecular Electrostatic potential plot of the title compound
Antimicrobial Activity 0f the Title Compound
The newly synthesized (2E)-3-(2,6-dichlorophenyl)-1-(4-4-Fluoro)-prop-2-en-1-one were screened for their antimicrobial activities in vitro against staphylococcus aureus, bacillus subtillis,Escheria coli salmonella Typhi pathogenic bacteria and three fungi Aspergillus Niger, Aspergillus flavus candida albicans. Antimicrobial activity of title compound was tested using the agar diffusion method [32].The antimicrobial activity data of synthesized compounds, from the result the title compound shows moderate activity against all organisms.
Table 6: Antimicrobial Activity
|
Product
|
Bacteria Fungi
|
|
Ec
|
Bs
|
St
|
Sa
|
An
|
Af
|
Ca
|
|
Compound
|
11(25)
|
12(25)
|
10(25)
|
09(25)
|
08(25)
|
12(25)
|
09(25)
|
|
Penicillin
|
15(25)
|
17(25)
|
13(25)
|
13(25)
|
NA
|
NA
|
NA
|
|
Nystatin
|
NA
|
NA
|
NA
|
NA
|
16(25)
|
14(25)
|
13(25)
|
|
Zone of inhibition is expressed in mm, Ec-Escherichia coli, An-Aspergillus niger, Sa-Staphylococcus aureus, Af-Aspergillus flavus, Bs- Bacillus subtillis-Salmonella Typhi, Ca- Candida albicans, — No activity, NA-Not Applicable
|
Conclusion
(2E)-3-(2, 6-dichlorophenyl)-1-(4-4-Fluoro)-prop-2-en-1-one synthesized by claisen-Schimdt condensation and spectroscopic characterization by using FT-IR and 1H NMR and study of molecular structure, bondlength, bond angle etc.by using Density Functional Theory B3LYP/6-311++G(d,p) basis set.It was found that experimental vibrational frequencies show good agreement with the calculated vibrational frequencies. The dipole moment of title compound is 3.9036 Debye shows polar nature. Allhydrogen’s are electropositive except H7 and C10 carries a higher negative charge. In addition to this energy of HOMO-LUMO, thermodynamic properties like Enthalpy, Entropy, and Polarizability are calculated. From the DFTstudy gives information about the reactivity of molecule.The title compound shows moderate activity against all bacteria and fungi.
Acknowledgements
The Authors acknowledge the central instrumentation facility, Savitribai Phule Pune University, Pune for NMR, KTHMCollege, and Nashik for FT-IR spectral analysis. The authors also would like to thank Principal of MGV’S L.V.H. Arts, Science and Commerce College, Panchvati, Nashik for permission and providing necessary research facilities. Authors are also grateful to Ex Professor A. B. Sawant for Gaussian study.Dr.Apoorva Prashant Hiray, Coordinator, MG Vidyamandir Institute, is gratefully acknowledged for Gaussian package.
Funding
No funding was received to carry out the research work presented in this research paper.
Conflict of interest
The author declares that they have no conflict of interest.
References
- Sharma SP, Vashisht N and Kumar S: Ultrasound promoted green synthesis of chalcones of 3-acetyl- coumarin. Chemical Sci Transactions 2018; 7(3): 396-01.
- 2. Al-AL Shaikh MA: Ultrasound-assisted Heterocycles Synthesis. Res. J. Chem. Environ 2016; 20(9): 36-46
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013; 2013:162750. Published 2013 Dec 29. doi:10.1155/2013/162750
CrossRef
- Mellado, M., Espinoza, L., Madrid, A. et al. Design, synthesis, antifungal activity, and structure–activity relationship studies of chalcones and hybrid dihydrochromane–chalcones. Mol Divers 24, 603–615 (2020). https://doi.org/10.1007/s11030-019-09967-y
CrossRef
- Umit Muhammet Kocyigit, Yakup Budak, Meliha Burcu Gürdere, Fatih Ertürk, Belkız Yencilek, Parham Taslimi, İlhami Gülçin & Mustafa Ceylan (2018) Synthesis of chalcone-imide derivatives and investigation of their anticancer and antimicrobial activities, carbonic anhydrase and acetylcholinesterase enzymes inhibition profiles, Archives of Physiology and Biochemistry, 124:1, 61-68, DOI: 10.1080/13813455.2017.1360914
CrossRef
- Deepa Gupta, D. K. Jain Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity J Adv Pharm Technol Res. 2015 Jul-Sep; 6(3): 114–117. doi: 10.4103/2231-4040.161507 PMCID: PMC454239
CrossRef
- Manik Das,Kuntal Manna, “Chalcone Scaffold in anticancer Armamentarium: A Molecular Insight”,Journal of Toxicology,vol.2016, 14pages,2016 ,https://doi.org/101155/2016/7651047
CrossRef
- Structure–Activity Relationships of Antileishmanial and Antimalarial Chalcones Mei Liu,a Prapon Wilairat,b Simon L. Croft,c Agnes Lay-Choo Tand and Mei-Lin Goa, Bioorganic & Medicinal Chemistry 11 (2003) 2729–2738
CrossRef
- Cole AL, Hossain S, Cole AM, Phanstiel O 4th. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg Med Chem. 2016 Jun 15;24(12):2768-76. doi: 10.1016/j.bmc.2016.04.045. Epub 2016 Apr 23. PMID: 27161874.
CrossRef
- Antimycobacterial and Anti-Inflammatory Activities of Substituted Chalcones Focusing on an Anti-Tuberculosis Dual Treatment Approach Thatiana Lopes Biá Ventura 1,2, Sanderson Dias Calixto 1 , Bárbara de Azevedo Abrahim-Vieira 3 , Alessandra Mendonça Teles de Souza 3 , Marcos Vinícius Palmeira Mello 4 , Carlos Rangel Rodrigues 3 , Leandro Soter de Mariz e Miranda 5 , Rodrigo Octavio Mendonça Alves de Souza 5, Ivana Correa Ramos Leal 3, Elena B. Lasunskaia 1,†,* and Michelle Frazão Muzitano 2,†,* Molecules 2015, 20, 8072-8093; doi:10.3390/molecules20058072
CrossRef
- Anandam, R., Jadav, S.S., Ala, V.B. et al. Synthesis of new C-dimethylated chalcones as potent antitubercular agents. Med Chem Res 27, 1690–1704 (2018). https://doi.org/10.1007/s00044-018-2183-z
CrossRef
- Sharma CS,Shekhawat,KS,Chauhan CS,Kumar N.,2013,Synthesis and anticonvulasant activity of some chalcone derivatives,Journal of chemical and pharmaceutical Research.5(10).pp.450-454.
- Nelly Mateeva, Suresh V. K. Eyunni, Kinfe K. Redda, Ucheze Ononuju, Tony D. Hansberry, II, Cecilia Aikens, Anita Nag Functional evaluation of synthetic flavonoids and chalcones for potential antiviral and anticancer properties, Bioorg Med Chem Lett. Author manuscript; available in PMC 2018 Jun 1.Published in final edited form as: Bioorg Med Chem Lett. 2017 Jun 1; 27(11): 2350–2356. . doi: 10.1016/j.bmcl.2017.04.034
CrossRef
- Sivakumar Pm,Prabhakar PK,Doble M (2011) Synthesis,anti-oxidant evaluation,and quantitative structure-activity relationship studies of chalcones.Med Chem Res20(4):480-492
CrossRef
- Sadgir, N.V., Dhonnar, S.L., Jagdale, B.S. Review on synthesis and biological activity of chalcone International Journal of Research and Analytical Reviews (IJRAR) February 2019, Volume 6, Issue 1
- Dhonnar S,Jagdale BS,Sawant AB,Pawar TB,Chobe SS(2016) Molecular structure,vibrational spectra and theoretical HOMO-LUMo analysis of (E) -3,5-dimethyl-1-phenyl-4-(p-tolyldiazenyl)-1H-pyrazole by DFT method.Der Pharma Chem 8(17):119-128
- M .J. Frisch, G.W. Trucks, H. B. Schlegel et. al. Gaussian 03, Revision C.02, Wallingford CT, Gaussian Inc.,2004.
- Hsieh CT,Hsieh TJ,EI-shazly m,Chuang DW,Tsai YH,Yen CT,Wu SF.Wu YC,Chang FR (2012), synthesis of chalcone derivatives as potential anti-diabetic agents.Biorg med Chem Lett 22(12):3912-3915
CrossRef
- Frisch, M. J. et al. Gaussian 03, Revision E.01, Gaussian, Inc., Wallingford CT, 2004.
- Lee, C.; Yang, W.; Parr, R. G., Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785-789, DOI: 10.1103/PhysRevB.37.785.
CrossRef
- Becke, A. D., Density-functional thermochemistry.III. The role of exact exchange. J. Chem. Phys. 1993,98, 5648-5652, DOI: 10.1063/1.464913.
CrossRef
- Roeges NPG (1994) A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures. Wiley, New York.
- . Zainuri DA, Arshad S, Khalib CN, Razak IA, Pillai RR, Sulaiman SF,.Hashim NS, Ooi K L, Armakovic S, Armakovic SJ, Panicker CY, Alsenoy CV (2017) Synthesis, XRDcrystal structure, spectroscopic characterization (FT-IR, 1H and 13C NMR), DFT studies,chemical reactivity and bond dissociation energy studies using molecular dynamicssimulations and evaluation of antimicrobial and antioxidant activities of a novel chalconederivative, (E)-1-(4-bromophenyl)-3-(4-iodophenyl)prop-2-en-1-one.J Mol Struct1128:520-533
CrossRef
- Maidur SR, Patil PS, Ekbote A, Chia TS,Quah CK (2017) Molecular structure, second and third-order nonlinear optical properties and DFT studies of a novel non centrosymmetric chalcone derivative: (2E)-3-(4-fluorophenyl)-1-(4-{[(1E)-(4-fluorophenyl)methylene]amino}phenyl)prop-2-en-1-one.Spectrochim.Acta.Part. A MoleBiomol Spectrosc 184:342-354
CrossRef
- Sadgir, N.V., Dhonnar, S.L., Jagdale, B.S. et al. Synthesis, spectroscopic characterization, XRD crystal structure, DFT and antimicrobial study of (2E)-3-(2,6-dichlorophenyl)-1-(4-methoxyphenyl)-prop-2-en-1-one.SN Appl.Sci. 2,1376(2020) DOI:10.1007/s42452-020-2923-9
CrossRef
- Koopmans, T.A.; on the assignment of wave functions and Eigen energies to the individual electrons of an atom. Physica, 1934,1,104-133,https://doi.org/10.1021/jo00267a034
CrossRef
- Pearson, R.G.; Absolute electronegativity and hardness: application to organic chemistry. J Org Chem.1989, 54, 1423-1430.
CrossRef
- Parr, R.G.; Sznetpaly, L.V.; Liu, S.J., Electrophilicity Index. J. Am. Chem. Soc. 1999, 121(9), 1922-1924,https://doi.org/10.1021/ja983494x.
CrossRef
- Chattaraj, P.K.; Giri, S., Stability reactivity and aromaticity of compound of multivalent superatom. J. Phys. Chem. A. 2007,111, 11116-11126,https://doi.org/10.1021/jp0760758.
CrossRef
- Chattaraj, P.K.; Maiti S.U., Philicity: A unified treatment of chemical reactivity and selectivity. J. Phys. Chem. A,2003, 107, 1089-5639, https://doi.org/10.1021/jp034707u
CrossRef
- S.S. Pathade and B.S. JagdaleExperimental and Computational Investigations on the Molecular Structure, Vibrational Spectra, Electronic Properties, FMO and MEP Analyses of 4,6-Bis(4- Fluorophenyl)-5,6-dihydropyrimidin-2(1H)-one: A DFT Insight Phys. Chem. Res., Vol. 8, No. 4, 671-687, December 2020 DOI: 10.22036/pcr.2020.227546.1763
- Shrinivasan D, Sangeetha N, Suresh 451 T,Lakshamanperumalsamy P (2001) Antimicrobial activity of certain Indian medicinal plants used folkloric medicines. J Ethnophrmacol74:217-220.
CrossRef
Views: 828
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 *, Sunil L.Dhonnar1, Bapu Jagdale2, Bhagyashri Waghmare3 and Chetan Sadgir4
*, Sunil L.Dhonnar1, Bapu Jagdale2, Bhagyashri Waghmare3 and Chetan Sadgir4 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal