Ganesh B. Yelmame1* and Shrikant B. Jagtap2
and Shrikant B. Jagtap2
1Department of Chemistry, Mahatma Gandhi Vidyamandir’s SPH.Arts, Science and Commerce College, (Affiliated to SP Pune University, Pune) Nampur, Nashik-423 204, India
2Departmen in Chemistry, Pune District Education Association’s Annasaheb Magar Mahavidyalaya, (Affiliated to Savitribai Phule Pune University, Pune) Hadapsar, Pune- 411028, India
Corresponding Author E-mail: yelmameganesh@gmail.com
DOI : http://dx.doi.org/10.13005/msri/180103
Article Publishing History
Article Received on : 20-Mar-2020
Article Accepted on : 14-Apr-2021
Article Published : 16 Apr 2021
Plagiarism Check: Yes
Reviewed by: Dr. Guru Swamy
Second Review by: Dr. Yogesh Chaudhari
Final Approval by: Dr. Ajay Mishra
Article Metrics
ABSTRACT:
Perimidines are available in an assortment of drugs and general use industrial structures and perimidines are also significant primary theme because of their extraordinary method of physiological activity. Thus the underlying significance of perimidine moiety has evoked a lot of interest in the field of natural blend and compound science to build up some better than ever amalgamation of this atomic skeleton. In this review, we have depicted a modern outline on the new advances in the different manufactured approaches of perimidine. The review covers the essential applied and down to earth synergist blend like, green methodologies, metal catalysed responses, microwave illumination, grinding and so forth which are critical for developing perimidine skeleton. This review will fulfil the assumptions for peruses who are keen on the advancement of the field and searching for an update. It will animate analysts to grow new and innovative manufactured admittance to this heterocyclic framework, which will be instrumental in the headway of perimidine science.This review provides an overview of various synthetic methodologies for the synthesis of a wide range of perimidine derivatives with applications in material chemistry, drug discovery, polymer chemistry, photo sensors, dye chemistry, and other fields.
KEYWORDS:
8 Diamino Naphthalene; Green Chemistry; Grinding; Perimidine
Copy the following to cite this article:
Yelmame G. B, Jagtap S. B. Review on Perimidines: A synthetic Pathways Approach. Mat. Sci. Res. India;18(1).
|
Copy the following to cite this URL:
Yelmame G. B, Jagtap S. B. Review on Perimidines: A synthetic Pathways Approach. Mat. Sci. Res. India;18(1). Available from: https://bit.ly/2PZKEQl
|
Introduction
Perimidine is synthesized by inserting a one-carbon unit between the nitrogen and closing the ring of 1,8-naphthalenediamine. Heteroaromatic structure displaying the distinct properties of compounds with abundance and deficit of electrons at the same time. Perimidine is one such framework, and its amphoteric chemical properties make it a fascinating research topic. Perimidine derivatives are explored in terms of polymer chemistry, drug discovery, photo sensors, dye industries, and catalytic action in organic synthesis 1. Perimidines and the pyrimidine fused with naptha framework is a relatively recent and rapidly expanding field of pure and applied chemistry. Structure, synthesis, spectral experiments, bonding with numerous motifs and ligands, andtheir varied reactivity in a variety of fields.Researchers are particularly interested in its environmentally friendly synthesis because of its unusual electronic properties and wide range of applications. Green Chemistry has emerged as new branch of chemistry for the synthesis of variety of compounds by employing green chemistry principles2-15. Various correspondences have been focussed in recent years to the biological activity of perimidines. Heterocyclic compounds were investigated to show wide of variety of biological properties 16-28. As a result of this concern, numerous perimidine and composite synthesis methodologies have been established. A significant number of perimidines have been designed under various conditions to date. Writing reports uncover that perimidines are of wide intrigue in view of their expansive range of biological activities[29-30]. Perimidines exhibit antihelminthic activity 31-33. Neurotropic active systems (stimulants and depressants of the central nervous system have been found out by using perimidines 34–36. Some compounds reveals good antitumor 37–40, antagonist41, antibacterial and antifungal42 activities. 2-(R’-oxymethyl)-perimidine derivatives have been proposed as highly effective antiulcer agent43. Some perimidine derivatives acts as antineoplastic agents44. Heterocyclic perimidines show antimicrobial45 and anorectic46 activity.The aim of the study is to highlight the most recent developments in perimidine synthesis under a variety of conditions.
Synthetic Pathways to Perimidines
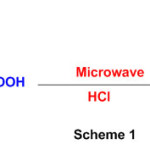
An acid-catalyzed reaction of carboxylic acids and 1,8-diaminonapthalene(Scheme 1) gives 2-substituted perimidine under microwave irradiation47.
Scheme 1
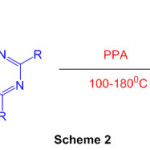
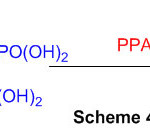
1,8-diaminonaphthalene reacts with 1,3,5-triazine in presence of polyphosphoric acid(Scheme 2) leads to perimidine 48.
Scheme 2
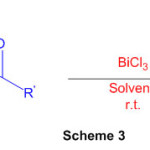
Various perimidine derivatives aresynthesized from naphthalene-1,8-diamine and variety of ketone functionality(Scheme 3) using catalyst BiCl3 49.
Scheme 3
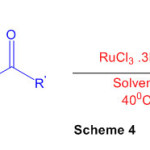
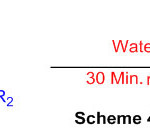
Perimidine can be synthesized using ruthenium (III) chloride (Scheme 4) under mild condition 50
Scheme 4
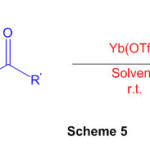
Different organically significant perimidine subordinates have been effectively orchestrated in amazing yields utilizing naphthalene-1,8-diamine with different ketones (Scheme 5) within the sight of a synergist measure of Yb(OTf)3 51
Scheme 5
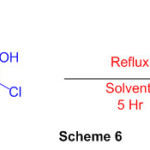
Another course to perimidines has been created which includes response of a 1,8-diaminonaphthalenewith chloro oxime derivative (Scheme 6) 52
Scheme 6
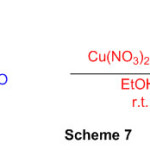
In the presence of a catalytic volume of Cu(NO3)2.6H2O in ethanol at room temperature, some perimidine subordinates is mixed by condensation reaction of 1,8 diaminonaphthalene and aromatic aldehydes. (Scheme 7) 53
Scheme 7
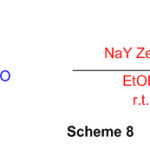
Perimidine is formed by reacting 1,8-diaminonaphthalene with aromatic aldehydes in the presence of NaY zeolite at room temperature. (Scheme 8) 54.
Scheme 8
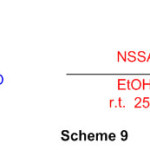
The cyclocondensationmethod of various aromatic aldehydes with 1,8diaminonaphthalene in the presence of nano-silica sulfuric acid (NSSA) as a catalyst has been used to create a reliable and direct method for the synthesis of perimidine subordinates. (Scheme 9) 55.
Scheme 9
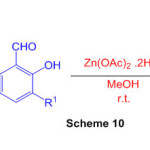
Perimidines are produced by reacting 1,8-diaminonaphthalene with aromatic aldehydes at room temperature in the presence of NaY zeolite. (Scheme 10) 56.
Scheme 10
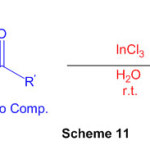
The reaction of naphthalene-1,8-diamine and active carbonyl compounds in water at room temperature showed InCl3 to be a mild and viable impetus for the simple and fruitful union of spiro-perimidine subsidiaries. (Scheme 11) 57.
Scheme 11
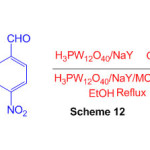
The study explores the catalytic activity of H3PW12O40/NaY and H3PW12O40/NaY/MCM-41 hybrid materials in the synthesis of perimidine (Scheme 12) 58.
Scheme 12
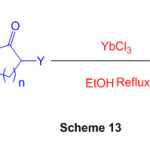
By building up aryl diamines with -carbonyl mixtures catalysed by ytterbium chloride, an effective technique for combining functionalized benzimidazoles and perimidines is developed (Scheme 13) 59.
Scheme 13
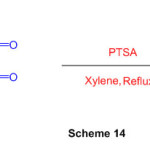
The multicomponent reaction of heterocyclic ketene aminals with dialkylacetylenedicarboxylates in the presence of DMAP is a suitable technique for the preparation of isoindole derivatives (Scheme 14)60.
Scheme 14
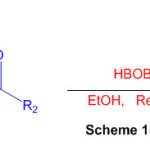
Using boric acid (HBOB) as a catalyst and ketone as a substrate, an efficient method for the synthesis of substituted perimidine derivatives is described (Scheme 15) 61.
Scheme 15
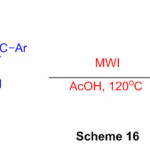
Another and proficient admittance to (Z)-N-(2-argio-1-(1H-perimidin-2-yl)vinyl)benzamide subsidiaries from promptly accessible substrates in HOAc is depicted with help of microwave irradiation (Scheme 16)62.
Scheme 16
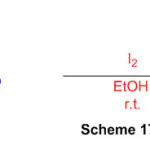
The reaction of 1,8-diaminonaphthalene and aromatic aldehydes in the presence of molecular iodine as a highly active catalyst yielded a few perimidines in high to exceptional yields (Scheme 17) 63.
Scheme 17
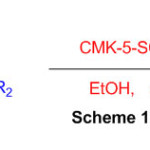
Using cyclocondensation of various aldehydes and ketones with 1,8-diaminonaphthalene in ethanol as a solvent at room temperature, sulfonated ordered nanoporous carbon effectively catalyses the synthesis of perimidines (Scheme 18) 64.
Scheme 18
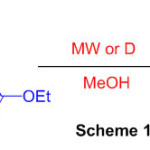
The reaction of 1,8-diaminonapthalene with iminoester hydrochlorides of substituted phenylacetic acids with microwave irradiation is identified for the synthesis of 2-substituted perimidines (Scheme 19) 65.
Scheme 19
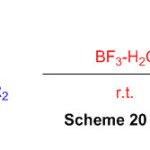
Synthesis of Perimidine and 1,5-Benzodiazepine Derivatives Using Tamed Brønsted Acid, BF3-H2O as a catalyst (Scheme 20) 66.
Scheme 20
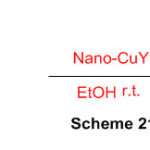
Another technique for union of 2-aryl-2,3-dihydro-1Hperimidines by buildup of 1,8-diaminonaphthalene with a variety of aromatic aldehydes utilizing nano-CuY zeolite as catalyst, in ethanol, at room temperature (Scheme 21) 67.
Scheme 21
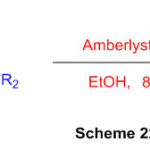
A proficient convention has been created to incorporate different dihydro-1H-perimidine subordinates utilizing financially accessible Amberlyst-15 as a catalyst (Scheme 22) 68.
Scheme 22
A successful union of different organically significant 2,3-dihydroperimidines from response of aldehydes and naphthalene-1,8-diamine has been created utilizing [BTBA] Cl-FeCl3 as an effective Lewis corrosive ionic fluid (Scheme 23) 69.
Scheme 23
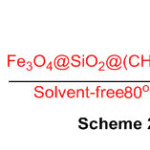
Functionalized magnetic center shell nanoparticles arranged by co-precipitation strategy, is a powerful and recyclable catalyst for the combination of imidazole, benzothiazole, and perimidine subsidiaries, under solvent free conditions (Scheme 24) 70.
Scheme 24
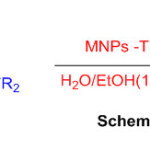
Nanoparticles as an efficient catalyst for synthesis of mono-, bis-, tris- and spiro-perimidines have been reported (Scheme 25) 71.
Scheme 25
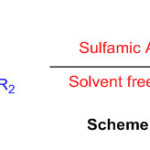
Gentle and relevant blend of mono-, bis-, and spiro-perimidines is exhibited in significant yields by means of the buildup of 1,8- diaminonaphthalene and aldehydes or ketones in presence of sulfamic acid as a green and exceptionally effective catalyst (Scheme 26) 72.
Scheme 26
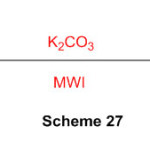
An easy immediate technique to blend the perimidine under dissolvable free conditions as another strategy is accounted under microwave irradiation (Scheme 27) 73.
Scheme 27
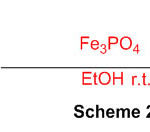
The response of 1,8-diaminonaphthalene with aromatic aldehydes gave 2-subbed perimidines within the sight of FePO4 as a flexible, green and reusable impetus at room temperature (Scheme 28)74.
Scheme 28
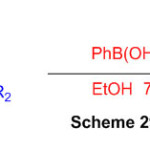
Synthesis of 2,3-dihydo-1H-perimidines subordinates was set up by considering a response between naphthalene-1,8-diamine and ketone within the sight of Phenyl boronic corrosive as an catalyst utilizing ethanol as a solvent (Scheme 29) 75.
Scheme 29
Exceptionally powerful heterogeneous impetus for improving the cyclocondensation response of 1,8-diaminonaphthalene with fragrant aldehydes to get 2,3-dihydro-1H-perimidine subsidiaries (Scheme30) 76.
Scheme30
A novel attractive nanocatalyst as a green, efficient and recoverable nanocatalyst pyrimidine subsidiaries could be effortlessly arranged utilizing this novel nanocatalyst in brilliant yields (Scheme 31) 77.
Scheme 31
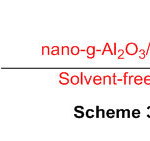
Nano-g-Al2O3/SbCl5 has been utilized for combination of 2-subbed perimidines by means of response of naphthalene-1,8-diamine with different aldehydes at room temperature under dissolvable free conditions (Scheme 32) 78.
Scheme 32
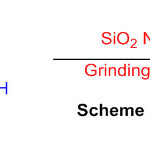
The reactant movement of SiO2 nanoparticles (NPs) as an eco-friendly, efficient and reusable impetus in the combination of 2,3-dihydro-1H-perimidines was found out (Scheme 33) 79.
Scheme 33
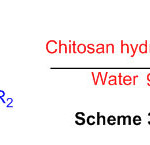
Proficient amalgamation of perimidine subordinates utilizing recently used chitosan hydrochloride was created (Scheme 34) 80.
Scheme 34
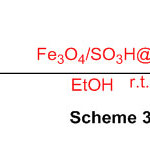
Fe3O4-stacked sulfonated zeolite was applied as a novel multi-functional zeolite impetus for the blend of perimidine subsidiaries (Scheme 35) 81.
Scheme 35
Fe3O4@NCs/BF0.2 was utilized for the amalgamation of 2,3-dihydro-1H-perimidine subsidiaries through a response of 1,8-diaminonaphthalene with different aldehydes at room temperature under dissolvable free conditions (Scheme 36) 82.
Scheme 36
The Mn-catalyzed dehydrogenative amalgamation of primarily significant 2,3-dihydro-1H-perimidines has been illustrated (Scheme 37) 83.
Scheme 37
The synergist execution of Ni/TCH@SBA‐15 (NNTS‐15) was resolved for the amalgamation of 2,3‐dihydroperimidines (Scheme 38)84.
Scheme 38
Hybrid nanomaterial was utilized as an effective impetus in the one-pot,green and straightforward convention for the combination of spiroperimidine subsidiaries (Scheme 39) 85.
Scheme 39
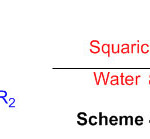
Squaric acid, a green, without metal and eco-accommodating organocatalyst, has been abused for the union of organically fascinating 2,3-dihydro-1H-perimidines (Scheme 40) 86.
Scheme 40
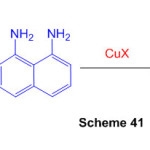
An epic amalgamation of profoundly combined perimidine subsidiaries was accomplished in two stages from 2-alkynylbenzaldehydes. Copper-catalyzed annulation of 2-[(2bromophenyl)ethynyl]benzaldehydes with 1,8-diaminonaphthalene created dihydroisoquinolino[2,1-a]perimidines (Scheme 41) 87.
Scheme 41
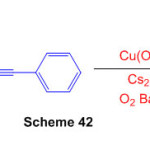
A Cu(OAc)2-catalyzed chain reaction tricyclization of naphthalene-1,8-diamine and 2-(phenylethynyl) benzaldehyde is described, allowing oxygen-consuming oxidative dehydrogenation coupling to heptacyclic quinolizin o[ 3,4,5,6-kla]perimidines to be obtained Scheme 42)88.
Scheme 42
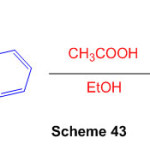
Spiro[1,3-dihydroperimidine-2,9-fluorene] and its co-crsytal with 9-fluoreone have been synthesized with the help of acetic acid as a catalyst (Scheme 43) 89.
Scheme 43
A few extremely successful one-pot manufactured methodswere established, allowing polyphosphoric acid activated nitroalkanes to act as electrophiles in aminonapthalene reactions. The methods illustrated consider the single-step set of polyheterocyclic aromatic subsidiaries of the 6H-pyrrolo[2,3,4-gh] perimidine platform in substantial returns. (Scheme 44) 90.
Scheme 44
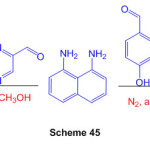
A active one-pot mix of two new heterocyclic perimidines, 4-(2,3-dihydro-1H-perimidin-2-yl)-2-methoxyphenol and 2-(2,3-dihydro-1H-perimidin-2-yl)-2-methoxyphenol and 2-(2,3-dihydro-1H-perimidin-2-yl)-2-meth (quinoxalin-2-yl) – Strong yields of 2,3-dihydro-1H-perimidine are produced. (Scheme 45)91.
Scheme 45
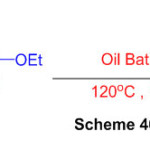
For the union of perimidines, another green protocol was established. The protocol involves a dissolvable and impetus-free reaction of ethoxycarbonylhydrazone with 1,8diaminonaphthalene. (Scheme 46) 92.
Scheme 46
The most environmentally sustainable method for mixing 2,3-dihydro-1H-perimidines with water is demonstrated. At room temperature, 1,8-diamino naphthalene was reacted with a variety of aldehydes to equip the item in low to high yields. (Scheme 47) 93.
Scheme 47
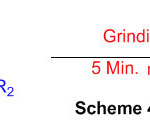
For the synthesis of 2,3-dihydro-1H-perimidines, benign, efficient, and green granulating assisted technique has been developed. The 1,8-diaminonaphthalene and aldehydes were ground for 5 minutes in a mortar and pestle, resulting in moderate to excellent yields (Scheme 48) 94.
Scheme 48
Conclusions
Perimidine synthesis remains a highly active area of research due to its diverse range of natural exercises, and its readiness will continue to be a significant task in the future. The development of manufactured routes for the preparation of perimidines has progressed in ways previously deemed challenging. In this report, an attempt was made to cover all of the major events and developments in science over the last few decades, which have seen exponential growth. These new reactions produce highly functionalized perimidines in good to excellent yields. A few approaches cover more recent techniques, cleaner synthetic substances that are not toxic to the environment, both of which lead to enhancing blend courses, reducing reaction projects, and making the relationship greener. Regardless of these developments, further work on designing new perimidine methods is expected. To offer pathways to the formation of neglected perimidines, new impetuses and synthetic improvements are needed, resulting in the disclosure of perimidines with new properties and natural exercises. We conclude this survey by hoping that it will inspire scientists to develop new and inventive manufactured access routes to this heterocyclic system, which will be critical in the advancement of many fields of research.
Acknowledgment
The authors acknowledge the Department of Chemistry, Mahatma Gandhi Vidyamandir’s SPH.Arts, Science and Commerce College, Nampur and Department of Chemistry, Pune District Education Association’s AnnasahebMagarMahavidyalaya, Hadapsar, Pune for providing necessary facilities.
Conflict of Interest
No potential conflict of interest was reported by the authors.
Funding Source
We have not received any kind of fund for the research work.
References
- Sahiba, N. and Agarwal, S., 2020. Recent Advances in the Synthesis of Perimidines and their Applications. Topics in Current Chemistry, 378(4), pp.1-47.
CrossRef
- Mandha, S.R., Siliveri, S., Alla, M., Bommena, V.R., Bommineni, M.R. and Balasubramanian, S., 2012. Eco-friendly synthesis and biological evaluation of substituted pyrano [2,3-c] pyrazoles. Bioorganic & medicinal chemistry letters, 22(16), pp.5272-5278.
CrossRef
- Desai, J.T., Desai, C.K. and Desai, K.R., 2008. A convenient, rapid and eco-friendly synthesis of isoxazoline heterocyclic moiety containing bridge at 2-amine as potential pharmacological agent. Journal of the Iranian Chemical Society, 5(1), pp.67-73.
CrossRef
- Adole, V.A., Waghchaure, R.H., Pathade, S.S., Patil, M.R., Pawar, T.B. and Jagdale, B.S., 2020. Solvent-free grindstone synthesis of four new (E)-7-(arylidene)-indanones and their structural, spectroscopic and quantum chemical study: a comprehensive theoretical and experimental exploration. Molecular Simulation, 46(14), pp.1045-1054.
CrossRef
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sagane, A.A., 2020. Ultrasound promoted stereoselective synthesis of 2,3-dihydrobenzofuran appended chalcones at ambient temperature. South African Journal of Chemistry, 73, pp.35-43.
CrossRef
- AbdEl‐Azim, M.H., Aziz, M.A., Mouneir, S.M., EL‐Farargy, A.F. and Shehab, W.S., 2020. Ecofriendly synthesis of pyrano [2, 3‐d] pyrimidine derivatives and related heterocycles with anti‐inflammatory activities. Archiv der Pharmazie, 353(9), p.2000084.
CrossRef
- Shinde, R.A., Adole, V.A., Jagdale, B.S., Pawar, T.B., Desale, B.S. and Shinde, R.S., 2020. Efficient Synthesis, Spectroscopic and Quantum Chemical Study of 2, 3-Dihydrobenzofuran Labelled Two Novel Arylidene Indanones: A Comparative Theoretical Exploration. Material Science Research India, 17(2), pp.146-161.
CrossRef
- Pourshojaei, Y., Jadidi, M.H., Eskandari, K., Foroumadi, A. and Asadipour, A., 2018. An eco-friendly synthesis of 4-aryl-substituted pyrano-fuzedcoumarins as potential pharmacological active heterocycles using molybdenum oxide nanoparticles as an effective and recyclable catalyst. Research on Chemical Intermediates, 44(7), pp.4195-4212.
CrossRef
- Adole, V.A., 2020. Synthetic approaches for the synthesis of dihydropyrimidinones/thiones (biginelli adducts): a concise review. World Journal of Pharmaceutical Research, 9(6), pp.1067-1091.
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2020. Aqua‐mediated rapid and benign synthesis of 1,2,6,7‐tetrahydro‐8H‐indeno[5,4‐b]furan‐8‐one‐appended novel 2‐arylidene indanones of pharmacological interest at ambient temperature. Journal of the Chinese Chemical Society, 67(2), pp.306-315.
CrossRef
- Mohebat, R., Yazdani-Elah-Abadi, A. and Simin, N., 2018. An efficient eco-friendly synthesis of pyran annulated heterocyclic systems under conventional heating and microwave irradiation in solvent-free conditions. Polycyclic Aromatic Compounds, 38(2), pp.180-188.
CrossRef
- Adole, V.A., Pawar, T.B., Koli, P.B. and Jagdale, B.S., 2019. Exploration of catalytic performance of nano-La2O3 as an efficient catalyst for dihydropyrimidinone/thione synthesis and gas sensing. Journal of Nanostructure in Chemistry, 9(1), pp.61-76.
CrossRef
- Adole, V.A., Bagul,V. R.,Ahire, S. A., Pawar,R. K.,Yelmame,G. B.,Bukane, A. R.,2019. Computational chemistry: molecular structure, spectroscopic (UV-visible and IR), electronic, chemical and thermochemical analysis of 3′-phenyl-1,2- dihydrospiro[indeno[5,4-b], Journal of Advanced Scientific Research, 12(1) Suppl 1, pp.276-286.
- Adole, V.A., Koli, P.B., Shinde, R.A. and Shinde, R.S., 2020. Computational Insights on Molecular Structure, Electronic Properties, and Chemical Reactivity of (E)-3-(4-Chlorophenyl)-1-(2-Hydroxyphenyl) Prop-2-en-1-one. Material Science Research India, 17(specialissue2020), pp.41-53.
CrossRef
- Shinde, R.A., Adole, V.A., Jagdale, B.S. and Pawar, T.B., 2020. Experimental and Theoretical Studies on the Molecular Structure, FT-IR, NMR, HOMO, LUMO, MESP, and Reactivity Descriptors of (E)-1-(2, 3-Dihydrobenzo [b][1, 4] dioxin-6-yl)-3-(3,4,5-trimethoxyphenyl) prop-2-en-1-one. Material Science Research India, 17(specialissue2020), pp.54-72.
CrossRef
- Chobe, S.S., Adole, V.A., Deshmukh, K.P., Pawar, T.B. and Jagdale, B.S., 2014. Poly (ethylene glycol)(PEG-400): A green approach towards synthesis of novel pyrazolo [3,4-d] pyrimidin-6-amines derivatives and their antimicrobial screening. Archives of Applied Science Research, 6(2), pp.61-66.
- Saini, M.S., Kumar, A., Dwivedi, J. and Singh, R., 2013. A review: biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res, 4(3), pp.66-77.
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2021. DFT computational insights into structural, electronic and spectroscopic parameters of 2-(2-Hydrazineyl) thiazole derivatives: a concise theoretical and experimental approach. Journal of Sulfur Chemistry, 42(2), pp.131-148.
- Gong, H.H., Addla, D., Lv, J.S. and Zhou, C.H., 2016. Heterocyclic naphthalimides as new skeleton structure of compounds with increasingly expanding relational medicinal applications. Current topics in medicinal chemistry, 16(28), pp.3303-3364.
CrossRef
- Adole, V.A., More, R.A., Jagdale, B.S., Pawar, T.B. and Chobe, S.S., 2020. Efficient synthesis, antibacterial, antifungal, antioxidant and cytotoxicity study of 2‐(2‐hydrazineyl) thiazole derivatives. ChemistrySelect, 5(9), pp.2778-2786.
- Pathade, S.S., Adole, V.A., Jagdale, B.S. and Pawar, T.B., 2020. Molecular structure, electronic, chemical and spectroscopic (UV-visible and IR) studies of 5-(4-chlorophenyl)-3-(3,4-dimethoxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazole: combined DFT and experimental exploration. Material Science Research India, 17(specialissue2020), pp.27-40.
- Herbert, J.M., Woodgate, P.D. and Denny, W.A., 1987. Potential antitumor agents. 53. Synthesis, DNA binding properties, and biological activity of perimidines designed as minimal DNA-intercalating agents. Journal of medicinal chemistry, 30(11), pp.2081-2086.
CrossRef
- Adole, V.A., Waghchaure, R.H., Jagdale, B.S. and Pawar, T.B., 2020. Investigation of Structural and Spectroscopic Parameters of Ethyl 4-(4-isopropylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: a DFT Study. Chemistry & Biology Interface, 10(1).
- Zhou, D.C., Lu, Y.T., Mai, Y.W., Zhang, C., Xia, J., Yao, P.F., Wang, H.G., Huang, S.L. and Huang, Z.S., 2019. Design, synthesis and biological evaluation of novel perimidine o-quinone derivatives as non-intercalative topoisomerase II catalytic inhibitors. Bioorganic chemistry, 91, p.103131.
CrossRef
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Desale, B.S., 2020. Molecular structure, frontier molecular orbitals, MESP and UV–visible spectroscopy studies of Ethyl 4-(3,4-dimethoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate: A theoretical and experimental appraisal. Material Science Research India, 17(specialissue2020), pp.13-36.
CrossRef
- Jain, K.S., Chitre, T.S., Miniyar, P.B., Kathiravan, M.K., Bendre, V.S., Veer, V.S., Shahane, S.R. and Shishoo, C.J., 2006. Biological and medicinal significance of pyrimidines. Current science, pp.793-803.
- Khopkar, S. and Shankarling, G., 2020. Squaric acid: an impressive organocatalyst for the synthesis of biologically relevant 2, 3-dihydro-1H-perimidines in water. Journal of Chemical Sciences, 132(1), pp.1-10.
CrossRef
- Adole, V.A., Waghchaure, R.H., Jagdale, B.S., Pawar, T.B. and Pathade, S.S., Molecular tructure, frontier molecular orbital and spectroscopic examination on dihydropyrimidinones: a comparative computational approach Journal of Advanced Scientific Research, 2020. 11 (2), pp.64-70.
- Bu, X., Deady, L.W., Finlay, G.J., Baguley, B.C. and Denny, W.A., 2001. Synthesis and cytotoxic activity of 7-oxo-7 H-dibenz [f, ij] isoquinoline and 7-oxo-7 H-benzo [e] perimidine derivatives. Journal of medicinal chemistry, 44(12), pp.2004-2014.
CrossRef
- Starshikov, N.M. and Pozharskii, F.T., 1973. Synthesis of 2-(5-halogeno-2-f uryl)-2, 3- dihydroperimidines. Chemistry of Heterocyclic Compounds, 9(7), pp.922-924.
CrossRef
- Drusvyatskaya, S.K., Lopatin, B.V., Bekhli, A.F., Krotov, A.I. and Naidenova, A.S., 1976. Vinyl derivatives of perimidine. Pharmaceutical Chemistry Journal, 10(5), pp.614-617.
CrossRef
- Bekhli, A.F., Drusvyatskaya, S.K., Lopatin, B.V., Naidenova, A.S. and Zelya, O.P., 1979. Conversions of perimidine 2-carbamates on acetylation and their anthelmintic properties. Pharmaceutical Chemistry Journal, 13(2), pp.161-163.
CrossRef
- Dasgupta, P.K., Lundquist, G.L., Reiszner, K.D. and West, P.W., 1977. Synthesis and application of perimidinylammonium bromide. AnalyticaChimicaActa, 94(1), pp.205-207.
CrossRef
- Komissarov, I.V., Konstantinchenko, A.A., Pozharskii, A.F., Filippov, I.T. and Kashparov, I.S., 1976. Synthesis and neurtropic activity of 2-aminoperimidines. Pharmaceutical Chemistry Journal, 10(7), pp.873-877.
CrossRef
- Pozharskii, A.F., Komissarov, I.V., Filippov, I.T., Konstantinchenko, A.A., Sheinkman, A.A. and Sokolov, V.I., 1977. Synthesis and neurotropic activity of 2-arylperimidines. Pharmaceutical Chemistry Journal, 11(5), pp.671-676.
CrossRef
- Dal’nikovskaya, V.V., Komissarov, I.V., Pozharskii, A.F. and Filippov, I.T., 1978. Synthesis and neurotropic activity of 1-acetonyl-and 1-phenacylperimidines. Pharmaceutical Chemistry Journal, 12(7), pp.899-902.
CrossRef
- Herbert, J.M., Woodgate, P.D. and Denny, W.A., 1987. Potential antitumor agents. 53. Synthesis, DNA binding properties, and biological activity of perimidines designed as minimal DNA-intercalating agents. Journal of medicinal chemistry, 30(11), pp.2081-2086.
CrossRef
- Dzieduszycka, M., Martelli, S., Arciemiuk, M., Bontemps-Gracz, M.M., Kupiec, A. and Borowski, E., 2002. Effect of modification of 6-[(aminoalkyl) amino]-7H-benzo [e]-perimidin-7-ones on their cytotoxic activity toward sensitive and multidrug resistant tumor cell lines. Synthesis and biological evaluation. Bioorganic & medicinal chemistry, 10(4), pp.1025-1035.
CrossRef
- Farghaly, T.A., Abbas, E.M., Dawood, K.M. and El-Naggar, T., 2014. Synthesis of 2- phenylazonaphtho [1, 8-ef][1, 4] diazepines and 9-(3-arylhydrazono) pyrrolo [1, 2-a] perimidines as antitumor agents. Molecules, 19(1), pp.740-755.
CrossRef
- Farghaly, T.A.E.R., Abdallah, M.A. and MMAHMOUD, H.K., 2015. Synthesis of novel 1, 2, 4-triazoles and triazolo-thiadiazines as anticancer agents. Turkish Journal of Chemistry, 39(5), pp.955-969.
CrossRef
- Luthin, D.R., Rabinovich, A.K., Bhumralkar, D.R., Youngblood, K.L., Bychowski, R.A., Dhanoa, D.S. and May, J.M., 1999. Synthesis and biological activity of oxo-7H-benzo [e] perimidine-4-carboxylic acid derivatives as potent, nonpeptidecorticotropin releasing factor (CRF) receptor antagonists. Bioorganic & medicinal chemistry letters, 9(5), pp.765-770.
CrossRef
- Panchasara, D.R. and Pande, S., 2009. Synthesis and Biological Activity of 3-Chloro-1-(4-perimidine methylcarbonylamino)-4-phenyl-azetidin-2-one. E-Journal of Chemistry, 6(S1), pp.S91-S96.
CrossRef
- Paragamian, V. and Taylor Jr, R.J., McNeilabInc, 1976. 2-(R’-oxymethyl)-perimidines. U.S. Patent 3,985,880.
- Stefanska, B., Dzieduszycka, M., Martelli, S., Tarasiuk, J., Bontemps-Gracz, M. and Borowski, E., 1993. 6-[(aminoalkyl) amino]-substituted 7H-benzo [e] perimidin-7-ones as novel antineoplastic agents. Synthesis and biological evaluation. Journal of medicinal chemistry, 36(1), pp.38-41.
CrossRef
- Farghaly, T.A., Abdallah, M.A. and Muhammad, Z.A., 2015. New 2-heterocyclic perimidines: synthesis and antimicrobial activity. Research on Chemical Intermediates, 41(6), pp.3937-3947.
CrossRef
- Liu, K.C., Chen, H.H. and Lin, Y.O., 1983. Synthe sis und Prufung von Thiazolo [3, 2-a] perimidin auf anorektische Wirkung. Arch. Pharm.(Weinheim), 316, pp.728-729.
CrossRef
- Mobinikhaledi, A., Foroughifar, N. and Goli, R., 2005. Synthesis of some benzotriazole-substituted perimidines . Phosphorus , Sulfur, and Silicon, 180(11), pp.2549-2554.
CrossRef
- Borovlev, I.V., Aksenov, A.V., Aksenova, I.V. and Pisarenko, S.V., 2007. 1, 3, 7-Triazapyrenes: the unexpected products of the reaction of 1, 8-diaminonaphthalene with 1, 3, 5-triazines in polyphosphoric acid. Russian Chemical Bulletin, 56(11), pp.2354-2355.
CrossRef
- Zhang, J. and Zhang, S., 2007. Bismuth (III) chloride–promoted efficient synthesis of perimidine derivatives under ambient conditions. Synthetic Communications, 37(15), pp.2615-2624.
CrossRef
- Zhang, J., Zhang, S.L. and Zhang, J.M., 2007. Ruthenium (III) chloride as an efficient catalyst for the synthesis of perimidine derivatives under mild conditions. Chinese Chemical Letters, 18(9), pp.1057-1060.
CrossRef
- Zhang, S.L. and Zhang, J.M., 2008. Ytterbium (III) triflate as an efficient catalyst for the synthesis of perimidine derivatives under mild conditions. Chinese Journal of Chemistry, 26(1), pp.185-189.
CrossRef
- Smellie, I.A., Fromm, A. and Paton, R.M., 2009. A new route to 2-substituted perimidines based on nitrile oxide chemistry. Tetrahedron Letters, 50(28), pp.4104-4106.
CrossRef
- Mobinikhaledi, A., Steel, P.J. and Polson, M., 2009. Rapid and efficient synthesis of Schiff bases catalyzed by copper nitrate. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 39(4), pp.189-192.
CrossRef
- Mobinikhaledi, A., FORUGHIFAR, N. and BASSAKI, N., 2009. Zeolite catalyzed efficient synthesis of perimidines at room temperature. Turkish journal of chemistry, 33(4), pp.555-560.
- Mobinikhaledi, A., Moghanian, H. and Sasani, F., 2010. A simple and convenient method for the synthesis of perimidine derivatives catalyzed by nano-silica sulfuric acid. International Journal of Green Nanotechnology: Physics and Chemistry, 2(2), pp.P47-P52.
CrossRef
- Belmonte, M.M., Escudero‐Adán, E.C., Benet‐Buchholz, J., Haak, R.M. and Kleij, A.W., 2010. Facile Synthesis of Substituted Mono‐, Di‐, Tri‐and Tetra‐2‐aryl‐2, 3‐dihydro‐1H‐perimidines.
CrossRef
- Yasaei, Z., Mirzaei, P. and Bazgir, A., 2010. InCl3-catalyzed efficient synthesis of spiro-perimidine derivatives . ComptesRendusChimie, 13(10), pp.1308-1312.
CrossRef
- Zendehdel, M., Mobinikhaledi, A., Alikhani, H. and Jafari, N., 2010. Preparation of heteropoly acid/porous hybrid materials and investigation of their catalytic behavior in the synthesis of perimidine. Journal of the Chinese Chemical Society, 57(4A), pp.683-689.
CrossRef
- Cai, L., Ji, X., Yao, Z., Xu, F. and Shen, Q., 2011. Efficient Synthesis of Functionalized Benzimidazoles and Perimidines: Ytterbium Chloride Catalyzed C-C Bond Cleavage. Chinese Journal of Chemistry, 29(9), pp.1880-1886.
CrossRef
- Üngören, Ş.H., Koca, I. and Yilmaz, F., 2011. Preparation of perinones via a novel multicomponent synthesis of isoindole scaffold. Tetrahedron, 67(30), pp.5409-5414.
CrossRef
- Phadtare, S.B., Vijayraghavan, R., Shankarling, G.S. and MacFarlane, D.R., 2012. Efficient synthesis of 2, 3-dihydro-1H-perimidine derivatives using HBOB as a novel solid acid catalyst. Australian Journal of Chemistry, 65(1), pp.86-90.
CrossRef
- Zhu, W.J., Ding, J., Jiang, B. and Tu, S.J., 2013. Highly efficient synthesis of tricyclic perimidines under microwave heating. Journal of Heterocyclic Chemistry, 50(S1), pp.E63-E66.
CrossRef
- Mobinikhaledi, A., Fard, M.B., Sasani, F. and Amrollahi, M.A., 2013. Molecular iodine catalyzed synthesis of some biologically active dihydroperimidines. BulgChemCommun, 45(3), pp.353-356.
- Alinezhad, H. and Zare, M., 2013. SULFONATED ORDERED NANOPOROUS CARBON AS AN EFICIENT SOLID CATALYST FOR THE SYNTHESIS OF PERIMIDINE DERIVATIVES. Journal of the Chilean Chemical Society, 58(3), pp.1840-1841.
CrossRef
- Kahveci, B., Karaali, N., Menteşe, E. and Yilmaz, F., 2013. Synthesis of new perimidine derivatives from the reaction of 1, 8-diaminonapthalene with iminoester hydrochlorides. Journal of Chemical Research, 37(6), pp.377-379.
CrossRef
- Prakash, G.S., Paknia, F., Narayan, A., Mathew, T. and Olah, G.A., 2013. Synthesis of perimidine and 1, 5-benzodiazepine derivatives using tamed Brønsted acid, BF3–H2O. Journal of Fluorine Chemistry, 152, pp.99-105.
CrossRef
- Kalhor, M. and Khodaparast, N., 2015. Use of nano-CuY zeolite as an efficient and eco-friendly nanocatalyst for facile synthesis of perimidine derivatives. Research on Chemical Intermediates, 41(5), pp.3235-3242.
CrossRef
- Patil, V.V. and Shankarling, G.S., 2014. A metal free, eco-friendly protocol for the synthesis of 2, 3-dihydro-1H-perimidines using commercially available Amberlyst 15 as a catalyst. Catalysis Communications, 57, pp.138-142.
CrossRef
- Bahrami, K. and Saleh, S., 2016. [BTBA] Cl-FeCl3 as an efficient Lewis acid ionic liquid for the synthesis of perimidine derivatives. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 46(6), pp.852-856.
CrossRef
- Farrokhi, A., Ghodrati, K. and Yavari, I., 2015. Fe3O4/SiO2/(CH2) 3N+ Me3Br3− core–shell nanoparticles: A novel catalyst for the solvent-free synthesis of five-and six-membered heterocycles. Catalysis Communications, 63, pp.41-46.
CrossRef
- Bodaghifard, M.A., Asadbegi, S. and Bahrami, Z., 2017. (Triazinediyl) bis sulfamic acid-functionalized silica-coated magnetite nanoparticles: Preparation, characterization and application as an efficient catalyst for synthesis of mono-, bis-, tris-and spiro-perimidines. Journal of the Iranian Chemical Society, 14(2), pp.365-376.
CrossRef
- Bodaghifard, M.A. and Ahadi, N., 2016. Sulfamic acid: A green and efficient catalyst for synthesis of mono-, bis-, and spiro-perimidines. Iranian Journal of Catalysis, 6(4), pp.377-380.
- Eldeab, H.A. and Eweas, A.F., 2018. A greener approach synthesis and docking studies of perimidine derivatives as potential anticancer agents. Journal of Heterocyclic Chemistry, 55(2), pp.431-439.
CrossRef
- Behbahani, F.K. and Golchin, F.M., 2017. A new catalyst for the synthesis of 2-substituted perimidines catalysed by FePO4. Journal of Taibah University for Science, 11(1), pp.85-89.
CrossRef
- Patil, V.D., Patil, K.P., Sutar, N.R. and Gidh, P.V., 2017. Phenyl boronic acid promoted efficient synthesis of perimidine derivatives under mild condition. ChemInt, 3(3), pp.195-201.
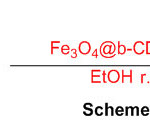
- Amrollahi, M.A. and Vahidnia, F., 2018. Decoration of β-CD-ZrO on Fe 3 O 4 magnetic nanoparticles as a magnetically, recoverable and reusable catalyst for the synthesis of 2, 3-dihydro-1 H-perimidines. Research on Chemical Intermediates, 44(12), pp.7569-7581.
CrossRef
- Ghodrati, K., Korani, E. and Asnaashari, M., 2018. Magnetic Core–Shell Nanoparticles Containing I3-as a Novel Catalyst for the Facial Synthesis of Imidazole Derivatives in Solvent-Free Conditions. Oriental Journal of Chemistry, 34(3), p.1504.
CrossRef
- Bamoniri, A., Mirjalili, B.B.F. and Saleh, S., 2018. Nano-γ-Al 2 O 3/SbCl 5: an efficient catalyst for the synthesis of 2, 3-dihydroperimidines. RSC Advances, 8(11), pp.6178-6182.
CrossRef
- Alinezhad, H., Ahmadi, A. and Hajiabbasi, P., 2019. Application of $$\hbox {SiO} _{{2}} $$ SiO 2 nanoparticles as an efficient catalyst to develop syntheses of perimidines and tetraketones. Journal of Chemical Sciences, 131(4), pp.1-10.
CrossRef
- Shelke, P.B., Mali, S.N., Chaudhari, H.K. and Pratap, A.P., 2019. Chitosan hydrochloride mediated efficient, green catalysis for the synthesis of perimidine derivatives. Journal of Heterocyclic Chemistry, 56(11), pp.3048-3054.
CrossRef
- Kalhor, M. and Zarnegar, Z., 2019. Fe 3 O 4/SO 3 H@ zeolite-Y as a novel multi-functional and magnetic nanocatalyst for clean and soft synthesis of imidazole and perimidine derivatives. RSC Advances, 9(34), pp.19333-19346.
CrossRef
- Mirjalili, B.B.F. and Imani, M., 2019. Fe3O4@ NCs/BF0. 2: A magnetic bio‐based nanocatalyst for the synthesis of 2, 3‐dihydro‐1H‐perimidines. Journal of the Chinese Chemical Society, 66(11), pp.1542-1549.
CrossRef
- Das, K., Mondal, A., Pal, D., Srivastava, H.K. and Srimani, D., 2019. Phosphine-free well-defined Mn (I) complex-catalyzed synthesis of amine, imine, and 2, 3-dihydro-1 H-perimidine via hydrogen autotransfer or acceptorlessdehydrogenative coupling of amine and alcohol. Organometallics, 38(8), pp.1815-1825.
CrossRef
- Kalhor, M., Rezaee‐Baroonaghi, F., Dadras, A. and Zarnegar, Z., 2019. Synthesis of new TCH/Ni‐based nanocomposite supported on SBA‐15 and its catalytic application for preparation of benzimidazole and perimidine derivatives. Applied Organometallic Chemistry, 33(5), p.e4784.
CrossRef
- Ahadi, N., Bodaghifard, M.A. and Mobinikhaledi, A., 2020. Preparation and characterization of a novel organic–inorganic hybrid nanostructure: application in synthesis of spirocompounds. Research on Chemical Intermediates, 46(7), pp.3277-3294.
CrossRef
- Khopkar, S. and Shankarling, G., 2020. Squaric acid: an impressive organocatalyst for the synthesis of biologically relevant 2, 3-dihydro-1H-perimidines in water. Journal of Chemical Sciences, 132(1), pp.1-10.
CrossRef
- Tokimizu, Y., Ohta, Y., Chiba, H., Oishi, S., Fujii, N. and Ohno, H., 2011. Direct synthesis of highly fused perimidines by copper (I)-catalyzed hydroamination of 2-ethynylbenzaldehydes. Tetrahedron, 67(29), pp.5168-5175.
CrossRef
- Feng, B.B., Liu, J.Q. and Wang, X.S., 2017. Cu (OAc) 2-catalyzed aerobic oxidative dehydrogenation coupling: Synthesis of heptacyclicquinolizino [3, 4, 5, 6-kla] perimidines. The Journal of organic chemistry, 82(3), pp.1817-1822.
CrossRef
- Li, Z. and Deng, W., 2011. Synthesis, characterization, crystal structure and DFT studies on 1′, 3′-dihydrospiro [fluorene-9, 2′-perimidine]. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy, 82(1), pp.56-62.
CrossRef
- Aksenov, A.V., Aksenov, N.A., Ovcharov, D.S., Aksenov, D.A., Griaznov, G., Voskressensky, L.G. and Rubin, M., 2016. Rational design of an efficient one-pot synthesis of 6H-pyrrolo [2,3,4-gh] perimidines in polyphosphoric acid. RSC advances, 6(85), pp.82425-82431.
CrossRef
- Varsha, G., Arun, V., Robinson, P.P., Sebastian, M., Varghese, D., Leeju, P., Jayachandran, V.P. and Yusuff, K.K.M., 2010. Two new fluorescent heterocyclic perimidines: first syntheses, crystal structure, and spectral characterization. Tetrahedron Letters, 51(16), pp.2174-2177.
CrossRef
- Menteşe, E., Yılmaz, F. and Kahveci, B., 2018. A new green protocol for the synthesis of 2-substituted perimidines from hydrazones under catalyst-and solvent-free conditions. Bulgarian Academy of Sciences.
- Harry, N.A., Cherian, R.M., Radhika, S. and Anilkumar, G., 2019. A novel catalyst-free, eco-friendly, on water protocol for the synthesis of 2, 3-dihydro-1h-perimidines. Tetrahedron Letters, 60(33), p.150946.
CrossRef
- Harry, N.A., Radhika, S., Neetha, M. and Anilkumar, G., 2020. A novel catalyst‐free mechanochemical protocol for the synthesis of 2, 3‐dihydro‐1 H‐perimidines. Journal of Heterocyclic Chemistry, 57(4), pp.2037-2043.
CrossRef
Views: 1,065
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 and Shrikant B. Jagtap2
and Shrikant B. Jagtap2 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal