Rezza Ruzuqi1* , Victor Danny Waas2 and Muhammad Ali Ulath1
, Victor Danny Waas2 and Muhammad Ali Ulath1
1Marine and Fisheries Polytechnic, Sorong City, Indonesia
2Pattimura University, Ambon, Indonesia
3Marine and Fisheries Polytechnic, Sorong City, Indonesia.
Corresponding Author E-mail: rezza_ruzuqi@yahoo.co.id
DOI : http://dx.doi.org/10.13005/msri/180211
Article Publishing History
Article Received on : 26 Apr 2021
Article Accepted on : 12 May 2021
Article Published : 20 May 2021
Plagiarism Check: Yes
Reviewed by: Dr. Akila  Second Review by: Dr. Syed Nasimul Alam
Second Review by: Dr. Syed Nasimul Alam  Final Approval by: Dr Raghu A V
Final Approval by: Dr Raghu A V
Article Metrics
ABSTRACT:
Microstructural analysis has been performed on magnesium alloy electrodes, the material used for saltwater lantern batteries. This research aims to obtain detailed and accurate information needed to support the analysis of magnesium alloy corrosion resistance caused by the electrolysis process using various analytical methods in SEM(Scanning Electron Microscopy). It is a tool that uses an electron beam to display the surface structure and composition of a test material. The test carried out on this magnesium alloy electrode is to crush the electrode into a fine powder. Then the powder is put into a container for SEM-EDS testing. Magnifications start from 1,000xuntil 15,000x. The results showed that the greater the magnification on the microscope, the more it was seen that the lumps looked brittle. Then on the surface of the magnesium alloy electrodes, 58.00 wt% magnesium material is contained.
KEYWORDS:
Microstructure; Magnesium Alloy Electrodes; SEM-EDS; Seawater Batteries
Copy the following to cite this article:
Ruzuqi R, Waas V. D, Ulath M. A. Microstructure Analysis of Magnesium Alloy Electrodes in Seawater Batteries. Mat. Sci. Res. India;18(2).
|
Copy the following to cite this URL:
Ruzuqi R, Waas V. D, Ulath M. A. Microstructure Analysis of Magnesium Alloy Electrodes in Seawater Batteries. Mat. Sci. Res. India;18(2). Available from: https://bit.ly/2RAjGzp
|
Introduction
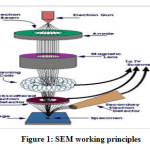
Electron microscopy is a modern tool that has changed the perspective of scientists about digital image technology, with the ability to characterize micro-sized to nano-sized materials. The Scanning Electron Microscope (SEM) is one of the most versatile modern instruments required for analyzing the microstructure and chemical composition of materials. This tool can see objects with a resolution of 10-100 nm, making this tool the choice of researchers in describing the surface morphology of the material.The basic working principle of the electron microscope begins with an understanding of the basic principles of light reflection. The working principle of SEM, as shown in Figure 1.Previously, there have been many scientific studies using light microscopy, especially for observing objects that are less than a micron in size.The working method of the electron microscope is to use a high-energy electron beam as a substitute for a light source. It originated from the discovery that electrons can be deflected by magnetic fields in 18901.
Figure 1: SEM working principles
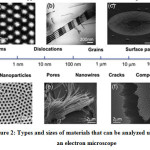
Electron Microscope can use on various types of materials, especially modern materials. Modern materials at the macro, micro, and even nano levels can easy characterized using it.Many complex composite materials evolved over thousands of years in nature. A modern manufacturing and fabrication method is needed to obtain a composite material with a structure, chemistry, and morphology tailored to the needs of the place where the material is applied.Figure 2.shows the types and sizes of materials that can be analyzed using an electron microscope. SEM can provide chemical information from specimens using a variety of techniques, including by equipping the X-ray energy-dispersive spectrometer (EDS).The use of SEM has also been applied in various sectors of life, including machinery 2, rock magnetism 3, porous materials 4, material for fuel cladding of nuclear reactor 5, and anything else.
Figure 2: Types and sizes of materials that can be analyzed using an electron microscope
Furthermore, apart from those used above, SEM equipment is also used to characterize brine batteries.In the Mg-Li alloy, deposits found using Scanning Electron Microscopy (SEM)6, Using microstructure observations, an anode activation of Mg alloys discussed 7, The surface morphology after discharge of Mg-Li alloysusing SEM 8, The surface morphology of the Mg-Gaalloycompared with AP65 alloy and AZ31 alloy 9,10,microstructure characterization behaviorof Mg-Alalloy 11, microstructure characterization of Ag electrodes 12, The microstructure behavior of a Mg-Al-Pb-La alloy 13,14, Microstructure characterization of hard carbon particle 15, microstructure characterization of Mg-6Al-1Sn-0.4Mn alloy anode 16, microstructure characterization of Co3V2O8 nanoparticles 17, microstructure characterization ofMg-Al-Zn-Ga alloys 18, microstructure characterization of Na super ion conductor (NASICON) solid electrolyte 19, and Activated carbon cloth and carbon felt investigated using SEM 20.
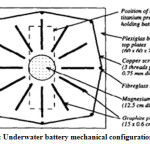
Seawater battery development began in 1996 when dissolved oxygen seawater batteries relied on reactive metal anode corrosion and oxygen reduction at the inert cathode to generate a potential of about 1 V when immersed in seawater.Underwater battery mechanical configuration design, as shown in Figure 3 21. The development of seawater batteries continues to date 20. It is to obtain energy storage technology for an environmentally friendly future from abundant and low-cost seawater for everyone to enjoy.
Figure 3: Underwater battery mechanical configuration design
In marine affairs and fisheries in general, the need for fishing support equipment or lighting tools when there is no electricity is principal. It is in line with efforts to develop appropriate and environmentally friendly alternative technologies to help coastal communities catch fish or do their daily activities. Then to support this, several researchers discovered and developed an environmentally friendly technology in the form of seawater lamps by reacting seawater with electrodes as a battery. In the development of seawater batteries, there are a variety of materials that are combined. It aims to create a product that has a high-efficiency value. Several types of magnesium alloy electrodes have been produced, including:Mg–Li alloys [6,8],Mg-Ga, AP65 alloy, and AZ31 alloy [9,10], Mg-Al alloy [11], Mg-Al-Pb alloy [13,14], Mg-Al-Sn-Mn alloy anode [16], and Mg-Al-Zn-Ga alloys [18]. Meanwhile, other materials can use to make seawater lamp batteries, including: Ag electrodes [12], hard carbon particle [14], Co3V2O8 nanoparticles [17], Na super ion conductor (NASICON) solid electrolyte [19], and Activated Carbon Cloth and Carbon Felt[20].
Then, to obtain detailed and accurate information needed to support the analysis of magnesium alloy corrosion resistance caused by the electrolysis process using various analytical methods in SEM-EDS from seawater lanterns that will be used by fishermen as a fishing aid.
Experimental and Procedures
Materials
In this research, the materials used were commercial product materials produced by LED Green Lantern. An example of a product as shown in Figure 4.This product is widely circulating in the market and is a commercial product widely used by saltwater lighting companies.
Figure 4: Magnesium alloy electrodes used in this study
Experiment
In this research, a magnesium alloy electrode crush into a fine powder. Then the powder is put into a container for SEM-EDS testing. The result is a topographic image and the elemental composition of the magnesium alloy electrode.
Results and Discussion
SEM and EDS testing are a series of tools used to analyze the topography and composition of a test material. Its working principle is to shoot an electron beam at the surface of the material. Then the electrons hit the sample and cause interactions between X-rays and electrons specific to the characteristics of each element seen and adjusted to the power table. So that the elements in the analyzed sample.
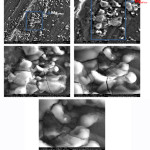
Figure 5: SEM test results of the magnesium alloy
Based on Figure 5, magnesium alloy is brittle and forms agglomerates that break down easily. Figure 5, shows that the magnification starts from 1,000xuntil 15,000x. The greater the magnification on the microscope, the more brittle magnesium alloy clumps seen. It is also the reason why magnesium decomposes easily in water, especially salt solutions. The nature of magnesium, which easily binds to the elemental chloride in NaCl, will increase the resulting potential difference.
Furthermore, it appears that the surface of the magnesium alloy electrode contains quite a lot of magnesium material, as shown in Figure 6. Magnesium is well known for being widely used in electrodes. This because the magnesium alloy material has a higher discharge current, but the utilization efficiency is lower when reacted in a NaCl-rich environment [8]. From this research, its hoped that in the future, there will be an alloy that will slightly increase its utilization efficiency when reacted in a NaCl-rich environment. It is related to the use of sea/salt water-fired lamp electrodes.
Figure 6: EDS test results of magnesium alloy
Then in the research used magnesium alloy electrodes produced by LED Green Lantern. Using the Energy Dispersive X-ray Spectroscopy (EDS) method, the chemical composition of the electrodes obtained.Figure 6, shows that the surface constituents of magnesium alloy are oxygen, magnesium, aluminum, and tin, with the largest percentage by weight of elemental magnesium at 58.00 wt%. These results prove that the material used in the manufacture of seawater/salt lamp electrodes is made from magnesium alloy with a composition of O = 38.63 wt%, Mg = 58.00 wt%, Al = 02.43 wt%, and Sn = 00.94. wt%.
Furthermore, magnesium is a material applied to seawater lantern batteries 16. From the surface morphology, it can be seen that several bright white particles of different sizes were identified as Mg by EDS testing. In this research, it has also been proven that in this magnesium alloy electrode material. It can be seen that there is a white lump which, after doing the EDS test proves that, the material is Mg with a weight percentage level of 58.00 wt%.
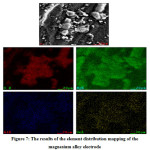
Figure 7: The results of the element distribution mapping of the magnesium alloy electrode
Then, to determine the distribution of elements in the magnesium alloy, element distribution mapping is used. The results of the mapping of the distribution elements are as shown in Figure 7. These results indicate the distribution of the elements Oxygen, Magnesium, Aluminum, and Tin. For the distribution of the element Oxygen, as shown in red, Magnesium as shown in green, Aluminum as shown in blue, and Tin as indicated by yellow.
Furthermore, based on Figure 7, the largest distributor of elements is Magnesium, Oxygen, Aluminum, and Tin. In the mapping results, the next dominant Oxygen. It indicates that Oxygen needs in the synthesis process of magnesium alloy. So that causes the amount of Oxygen in the magnesium alloy.
Conclusions
The development of environmentally friendly technology, namely seawater lanterns, is to help the fishermen and coastal communities as a means of lighting when fishing or their daily needs. As it that the fishermen and coastal communities in remote areas have electricity resource less.
Furthermore, for the SEM-EDS test results, the excellent surface morphology of magnesium alloy material was obtained. There are many pitting points, so the corrosion is more uniform. And the potential sample means discharge increases when varied with different salinity values. From the SEM-EDS results also, the constituent elements of this magnesium alloy are Oxygen, Magnesium, Aluminum, and Tin with the respective weight percentages of O = 38.63 wt%, Mg = 58.00 wt%, Al = 02.43 wt%, and Sn = 00.94 wt% obtained.
Acknowledgement
This work was supported by the Directorate of Research Marine and Fisheries Polytechnic of Sorong and Energy Laboratory of Materials and Metallurgy Engineering Study Program Surabaya Sepuluh Nopember Institute of Technology.
Funding Source
This research received no specific grant from any funding agency. The funding is done by author himself.
Conflict of Interests
The authors have no conflicts of interest to disclose.
References
- O. C. Wells. (1974): Scanning Electron Microscopy, McGraw-Hill, New York.
- Kardiman, K., Marno, M., &Sumarjo, J.,Analisi sSifat Mekanik Terhadap Bentuk Morfologi Papan Komposit SekamPadiSebagai Material AlternatifPenggantiSeratKaca, JRST (JurnalRisetSainsdanTeknologi), 2, (1), 21-26(2018).
CrossRef
- Anggraeni, N. D., & No, J. P. M.,Analisa SEM (Scanning Electron Microscopy) dalamPemantauan Proses Oksidasi Magnetite Menjadi Hematite, 7, 50-56 (2008).
- Chen, J., Xiao, Guangchun., Duan, Gaigai., Wu, Yongzhong., Zhao, Xiujian., & Gong, Xiao., Structural design of carbon dots/porous materials composites and their applications,Chemical Engineering Journal, 72, 1-71 (2020).
CrossRef
- Sujatno, A., Salam, R., Bandriyana, B., &Dimyati, A., STUDI SCANNING ELECTRON MICROSCOPY (SEM) UNTUK KARAKTERISASI PROSES OXIDASI PADUAN ZIRKONIUM,Jurnal Forum Nuklir, 9, 1, 44-50(2015).
CrossRef
- Yan, Y. D., Zhang, M. L., Han, W., Cao, D. X., Yuan, Y., Xue, Y., & Chen, Z., Electrochemical formation of Mg–Li alloys at solid magnesium electrode from LiCl–KCl melts,ElectrochimicaActa, 53, 8, 3323–3328(2008).
CrossRef
- Hongyang, Z., Pei, B., &Dongying, J., Electrochemical performance of magnesium alloy and its application on the sea water battery,Journal of Environmental Sciences Supplement, 4, S88-S91 (2009).
CrossRef
- Cao, D., Cao, X., Wang, G., Wu, L., & Li, Z., Electrochemical discharge performance of Mg-Li based alloys in NaCl solution, J Solid State Electrochem, 5, 14, 851-855 (2010).
CrossRef
- Zhao, J., Yu, K., Hu, Y., Li, S., Tan, X., Chen, F., & Yu, Z., Discharge behavior of Mg–4 wt%Ga–2 wt%Hg alloy as anode for seawater activated battery,ElectrochimicaActa, 56, 8224-8231 (2011).
CrossRef
- Yu, K., Huang, Q., Zhao, J., & Dai, Y., Electrochemical properties of magnesium alloy anodes discharged in seawater, Transactions of Nonferrous Metals Society of China, 22, 9, 2184–2190 (2012).
CrossRef
- Wen, L., Yu, Kun., Xiong, Hanqing., Dai, Yilong., Yang, Shihai., Qiao, Xueyan., Teng, Fei.,& Fan, Sufeng., Composition optimization and electrochemical properties of Mg-Al-Pb-(Zn) alloys as anodes for seawater activated battery, ElectrochimicaActa,194, 40-51 (2012).
CrossRef
- Kim, K., Hwang, S. M., Park, J.-S., Han, J., Kim, J., & Kim, Y., Highly improved voltage efficiency of seawater battery by use of chloride ion capturing electrode, Journal of Power Sources, 313, 46–50 (2016).
CrossRef
- Shi, Yinchun., Peng, Chaoqun., Feng, Yan., Wang, Richu., & Wang, Naiguang., Enhancement of discharge properties of an extruded Mg-Al-Pb anode for seawater-activated battery by lanthanum addition, Journal of Alloys and Compounds, 721, 392-404 (2017).
CrossRef
- Shi, Y., Peng,Chaoqun., Feng, Yan., Wang, Richu., & Wang, Naiguang., Microstructure and electrochemical corrosion behavior of extruded Mg-Al-Pb-La alloy as anode for seawater-activated battery, Materials and Design, 124, 24-33 (2017).
CrossRef
- Kim, Y. Kim, Jae-Kwang., Vaalma, Christoph., Bae, GeunHyeong., Kim, Guk-Tae., Passerini, Stefano., & Kim, Youngsik., Optimized hard carbon derived from starch for rechargeable seawater batteries, Carbon, 129, 564-571 (2018).
CrossRef
- Zheng, T., Hu, Y., Zhang, Y., Yang, S., & Pan, F., Composition optimization and electrochemical properties of Mg- Al-Sn-Mn alloy anode for Mg-Air batteries, Materials & Design,137, 245-255 (2018).
CrossRef
- Shin, K. H., Park, J., Park, S. K., Nakhanivej, P., Hwang, S. M., Kim, Y., & Park, H. S., Cobalt vanadate nanoparticles as bifunctional oxygen electrocatalysts for rechargeable seawater batteries, Journal of Industrial and Engineering Chemistry, 72, 250–254 (2019).
CrossRef
- Li, J., Chen,Zhuoyuan, Jing, Jiangping, &Hou, Jian.,Electrochemical behavior of Mg-Al-Zn-Ga-In alloy as the anode for seawater-activated battery, Journal of Materials Science & Technology, 41, 33-42 (2020).
CrossRef
- Bae, H., Park, Jeong-Sun, Senthilkumar, S.T., Hwang, Soo Min, & Kim, Youngsik., Hybrid seawater desalination-carbon capture using modified seawater battery system, Journal of Power Sources, 410-411, 99-105 (2019).
CrossRef
- Park, J., Park, Jeong-Sun., Senthilkumar, S.T., & Kim, Youngsik.,Hybridization of cathode electrochemistry in a rechargeable seawater battery: Toward performance enhancement, Journal of Power Sources, 450,227600 (2020).
CrossRef
- Wilcock, William S.D., & Kauffman, Peter C., Development of a seawater battery for deep-water applications, Journal of Power Source, 66, 71-75 (1997).
CrossRef
Views: 1,060
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 , Victor Danny Waas2 and Muhammad Ali Ulath1
, Victor Danny Waas2 and Muhammad Ali Ulath1

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal