Introduction
Pietro Biginelli reported the Biginelli reaction, a three-component reaction, in 1893. P. Biginelli reported the acid-catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) through a multicomponent reaction (MCR) of an aromatic aldehyde, ethyl acetoacetate, and urea. The Biginelli adduct is formed in the classic version of this reaction, which involves an acid-catalyzed three-component reaction between benzaldehyde, ethylacetoacetate, and urea in ethanol at reflux. DHMPs and related compounds demonstrate potential in the treatment of cancer 1-3, calcium channel inhibition 4-5, antioxidant 6, 7, antimicrobial 8-10, and anti-inflammatory function 11. The noteworthy medicinal profile of the DHPMs has prompted the synthesis of new Biginelli reaction methodologies.
In the past few years, researchers have focused on green concepts in order toto establish environmentally sustainable synthetic methodologies for synthesising a wide range of organic compounds12-21. Lewis acid catalysis 22, polymer-supported 23, ionic liquids 24, microwave-assisted synthesis 25, solvent-free techniques 26, 27, and other similar methods have all been published in the last few years.However, most reported dihydropyrimidinone synthesis strategies tohave drawbacks such as intolerant reaction conditions, long reaction times, unsatisfactory yields, higher reaction temperatures, and the use of expensive catalysts that are not recognized as environmentally friendly.
The metal oxide NPs are thought to be more reactive as a catalyst because they have a larger surface area that is more readily accessible to substrate molecules, resulting in better catalytic action 28, 29. Nanocatalysts have exceptional properties due to their high surface area to volume ratio, which makes them superior to mass materials in terms of catalysis.To achieve organic reactions in a short amount of time with higher yields, a variety of nano-catalysis techniques have been used 30-32. The ZnO NPs were reported as anexcellent catalysts for gas sensing 33, 34, multicomponent reactions 35-37 and dye degradation 38, 39 applications.Following that, here an attempt is made to develop a better catalyst for the synthesis of dihydropyrimidinones that was both easy to use and rendered high yields. On this basis, the present study reports ZnONPs as an effective catalyst for the synthesis of dihydropyrimidinones.
Experimental
Materials
Zn(NO3)2•6H2O (purity: >98%), NaOH (purity: >98%), aromatic aldehydes (purity: >97%), ethyl acetoacetate(purity: >97%),acetylacetone (purity: >97%), urea(purity: >98%), and thiourea (purity: >97%), were purchased from Sigma Aldrich, SDFCL and Avra synthesis and were used without any further purification. Melting points were determined in open capillaries and are uncorrected. 1H NMR and 13C NMR spectra were recorded with a Bruker using CDCl3solvent. The powder X-Ray Diffraction (XRD) technique was used to investigate the average crystallite size of the prepared nanomaterials on aBruker D8 AdvanceX-Ray Diffraction instrument.
Synthesis and characterization of ZnONPs
The co-precipitation method was used to produce the ZnO NPs. 1M of Zn(NO3)2•6H2O was dissolved in distilled water and the solution was kept under constant stirring usinga magnetic stirrer for 30minutes. Following full dissolution, a 2M sodium hydroxide solution was added while stirring constantly. The reaction was allowed to continue for two hours. The white solution was produced at the end of the reaction and was allowed to settle for 12 hours.The precipitate was then washed with distilled water several times before being dried in an oven. In a muffle furnace, the obtained product was kept at 400 °C for 3 hours to obtain ZnONPs.
General procedure for preparation of 3,4-dihydropyrimidine-2(1H)-ones/thiones
A mixture of aromatic aldehyde (0.01mol), urea/thiourea (0.01mol), and ethyl acetoacetate/acetylacetone (0.01mol) were taken in a flat bottom flask. 15 mL methyl alcohol was added to the solvent, and the resulting mixture was stirred until a clear solution was obtained. Then ZnONPs(10 mol %) wasadded. This mixture was heated on a magnetic stirrer at 80°C with continuous stirring and then it was allowed to cool at room temperature after completion of thereaction (monitored by TLC). Afterward, ethyl acetate was introduced and the extract was processed to acquire crude products free of ZnO NPs. The ethyl acetate extract was dried over sodium sulphate before being evaporated with a rotary evaporator.Spectroscopic data such as 1HNMR and 13C NMR spectral data were used to validate the structure of all synthesized products.
Scheme 1: ZnONPs catalyzed dihydropyrimidine-2(1H)-one/thione synthesis
Physicochemical and Spectral data of the selected compounds
6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Yield: 96 %, Colour: white solid, m.p. : 232 °C; 1HNMR (400MHz, DMSO-d6) δ (ppm): 1.10 (t, J = 7.1 Hz, 3H), 2.27 (s, 3H), 4.00 (q, J = 7.1 Hz, 2H), 5.14 (s, 1H), 7.27-7.35 (m, 5H), 7.75 (s, 1H), 9.18 (s, 1H); 13C NMR (100 MHz, DMSO-d6); δ (ppm) 14.54, 18.24, 54.43, 59.65, 99.73, 126.71, 127.73, 128.86, 145.34, 148.83, 152.60, 165.81.
Ethyl-4-(4-isopropylphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Yield: 90 %, Colour: white solid, m.p. : 176 °C; 1HNMR (400MHz, DMSO-d6); δ (ppm):1.19 (t, J = 7.1 Hz, 3H) 1.23 (d, J = 7.2 Hz, 6H), 2.33 (s, 3H), 2.85 (sept, J = 7.2 Hz, 1H), 4.07 (q, J = 7.1 Hz, 2H), 5.37 (d, 1H), 5.91 (s, 1H), 7.17(d, J = 8.2 Hz, 2H), 7.25 (d, J = 8.2 Hz, 2H), 8.55 (s, 1H);13C NMR (100 MHz, DMSO-d6) δ (ppm):14.54, 18.24, 54.43, 59.65, 99.73, 126.71, 127.73, 128.86, 145.34, 148.83, 152.60, 165.81.
5-acetyl-4-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one
Yield: 92 %, Colour: white solid, m.p. : 220 °C; 1HNMR (400MHz, DMSO-d6); δ (ppm): 2.11 (s, 3H), 2.30 (s, 3H), 5.25 (d, J = 3.8Hz, 1H), 7.40 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 9.78 (s, 1H), 10.30 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 18.85, 31.07, 53.53, 110.85, 128.94, 129.15, 132.78,142.33, 145.49, 174.72, 195.19.
Ethyl-4-(4-hydroxy-3-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Yield: 89 %, Colour: white solid, m.p. : 232 °C; 1HNMR (400MHz, DMSO-d6) δ (ppm): 1.09 (t, J = 7.2 Hz, 3H). 2.20 (s, 3H), 3.96 (q, J = 7.2 Hz, 2H), 6.75 (d, J = 2.1 Hz, 1H), 5.03 (d, J = 3.4 Hz, 1H), 6.67 (d, J = 8.1 Hz, 1H), 6.57 (dd, J = 8.1, 2.1 Hz, 1H), 8.89 (s, 1H), 9.09 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ (ppm):14.68, 18.26, 54.06, 56.05, 59.65, 100.04, 111.35, 115.78, 118.78, 136.43, 146.29, 147.75, 148.44, 152.76, 165.97
Result and Discussion
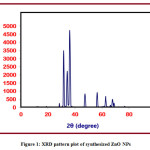
Powder XRD is an analytical method which is used to determine the phase of a crystallite substance and can also be used to determine unit cell dimensions. The hexagonal phase is visible in the XRD pattern of the synthesized ZnONPs (Figure 1). The synthesized ZnONPs showed sharper and stronger diffraction peaks, according to the XRD spectrum. The diffraction peaks at 31.65°, 34.32°, 36.14°, 47.43°, 56.46°, 62.74°, 66.18°, 67.79°, and 68.91° are correlated with the (100), (002), (101), (102), (110), (103), (200), (112), and (201) planes, respectively, and are mentioned in Table 1.The (hkl) values are in good agreement with the ZnO standard cards (JCPDS file No: 79-2205). Debye Scherrer’s formula is used to calculate the average crystallite size of the sample (D): D = 0.9λ/βcosθ, where, λ is the wavelength of the X-ray radiation, θ is the diffraction angle and β is the full width half maximum (FWHM) intensity.From the line broadening of the X-ray diffraction peak, the crystallite size of the NPs was determined using the formula above. The average crystallite size is 25 nm.
Figure 1: XRD pattern plot of synthesized ZnO NPs
Table 1: The observed and standard 2θ values of XRD data of synthesized ZnONPs
|
Observed 2θ
|
h kl
|
|
31.65
|
100
|
|
34.32
|
002
|
|
36.14
|
101
|
|
47.43
|
102
|
|
56.46
|
110
|
|
62.74
|
103
|
|
66.18
|
200
|
|
67.79
|
112
|
|
68.91
|
201
|
All of the experiments were carried out with an optimized catalyst concentration (Table 2). In a comparative study of the typical reaction of benzaldehyde, ethyl acetoacetate, and urea at 80 °C, the best result was obtained using a catalyst concentration of 10 mol percent. There was no apparent loss of catalytic activity after recycling and reusing the catalyst for three runs in a phase.Surprisingly, I found that different substitution patterns of benzaldehydes produced similar amounts of 3,4-dihydropyrimidine-2(1H)-ones/thiones (Table 3). The disclosed method stands out as compared to previous synthetic approaches. (Table 4).
Table 2: Optimization of reaction conditions
|
Entry
|
Catalyst
(mol %)
|
Temperature (°)
|
Time
(min)
|
Yield
(%)
|
|
1
|
–
|
rt
|
300
|
n.r.
|
|
2
|
–
|
60
|
300
|
n.r.
|
|
3
|
–
|
70
|
300
|
n.r.
|
|
4
|
–
|
80
|
300
|
10
|
|
5
|
5
|
rt
|
300
|
n.r.
|
|
6
|
5
|
60
|
300
|
n.r.
|
|
7
|
5
|
70
|
180
|
70
|
|
8
|
5
|
80
|
180
|
75
|
|
9
|
10
|
rt
|
300
|
n.r.
|
|
10
|
10
|
60
|
120
|
70
|
|
11
|
10
|
70
|
80
|
87
|
|
12
|
10
|
80
|
60
|
96
|
Reactions were carried out on 0.01 mol scale of all three reactant molecules;n.r. = no reaction; rt = room temperature.
Table 3: Physicochemical data of synthesized compounds
|
Entry
|
R1
|
R2
|
X
|
Time
(min)
|
Yield (%)*
|
Melting point (°C)
|
Reference
|
|
4a
|
OEt
|
–
|
O
|
60
|
96
|
232
|
13
|
|
4b
|
OEt
|
4-Cl
|
O
|
50
|
97
|
227
|
13
|
|
4c
|
CH3
|
4-N(CH3)2
|
O
|
85
|
91
|
242
|
13
|
|
4d
|
CH3
|
4-N(CH3)2
|
S
|
90
|
89
|
214
|
13
|
|
4e
|
CH3
|
4-Cl
|
O
|
65
|
92
|
220
|
13
|
|
4f
|
CH3
|
4-Cl
|
S
|
70
|
90
|
219
|
13
|
|
4g
|
OEt
|
4-CH(CH3)2
|
O
|
82
|
90
|
176
|
13
|
|
4h
|
OEt
|
4-OH, 3-OCH3
|
O
|
90
|
89
|
232
|
13
|
|
4i
|
CH3
|
4-OH, 3-OCH3
|
S
|
82
|
88
|
222
|
13
|
|
4j
|
CH3
|
3-OCH3, 4-OCH3, 5- OCH3
|
S
|
75
|
88
|
215
|
13
|
* Yield refers to pure isolated products
Table 4: Comparison between reported and present synthetic methodology for the synthesis of 4a
|
Entry
|
Reaction Conditions
|
Yield
|
Reference
|
|
1
|
PEG-400/Reflux
|
85
|
40
|
|
2
|
Sulfamic acid/70-80℃
|
78
|
41
|
|
3
|
Water (3-4 drops)/80℃
|
92
|
42
|
|
4
|
Co(NO3)2.6H2O/80℃
|
90
|
43
|
|
5
|
SiO2-Cl/80℃
|
88
|
44
|
|
6
|
PEG-400/Stirring
|
85
|
40
|
|
7
|
PEG-400/Ultrasound
|
92
|
40
|
|
8
|
PEG-400/Microwave
|
92
|
40
|
|
9
|
Organoclay/Water/Reflux
|
89
|
45
|
|
10
|
ZnO NPs/Methanol/80℃
|
96
|
Present method
|
Conclusion
The catalytic activity of ZnO NPs in the Biginelli reaction for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives was examined, and it was found that these ZnO NPs furnished dihydropyrimidinones with remarkable efficiency. A short reaction time, the use of a cost-effective, non-toxic, green catalyst, and clean reaction transformation with excellent yields are among the protocol’s key highlights.
Acknowledgments
Authoris grateful to the Prin. Dr. A.V. Patil, Mahatma Gandhi Vidyamandir’s Arts, Science and Commerce College, Surgana for providing research facilities. SAIF, Punjab University, Chandigarh is acknowledged for providing spectral analysis. Authoris expressing their sincere thanks to Dr. Aapoorva P. Hiray, Coordinator, Mahatma Gandhi Vidyamandir Institute, Nashik for his generous support.
Conflict of Interest
The author declares that he do not have any conflict of interest.
Funding
No funding was received to carry out the research presented in the paper.
References
- Mayer, T.U., Kapoor, T.M., Haggarty, S.J., King, R.W., Schreiber, S.L. and Mitchison, T.J., 1999. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science, 286(5441), pp.971-974.
CrossRef
- Wright, C.M., Chovatiya, R.J., Jameson, N.E., Turner, D.M., Zhu, G., Werner, S., Huryn, D.M., Pipas, J.M., Day, B.W., Wipf, P. and Brodsky, J.L., 2008. Pyrimidinone-peptoid hybrid molecules with distinct effects on molecular chaperone function and cell proliferation. Bioorganic & medicinal chemistry, 16(6), pp.3291-3301.
CrossRef
- Kumar, B.P., Sankar, G., Baig, R.N. and Chandrashekaran, S., 2009. Novel Biginelli dihydropyrimidines with potential anticancer activity: a parallel synthesis and CoMSIA study. European journal of medicinal chemistry, 44(10), pp.4192-4198.
CrossRef
- Atwal, K.S., Rovnyak, G.C., Schwartz, J., Moreland, S., Hedberg, A., Gougoutas, J.Z., Malley, M.F. and Floyd, D.M., 1990. Dihydropyrimidine calcium channel blockers: 2-heterosubstituted 4-aryl-1, 4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. Journal of medicinal chemistry, 33(5), pp.1510-1515.
CrossRef
- Atwal, K.S., Rovnyak, G.C., Kimball, S.D., Floyd, D.M., Moreland, S., Swanson, B.N., Gougoutas, J.Z., Schwartz, J., Smillie, K.M. and Malley, M.F., 1990. Dihydropyrimidine calcium-channel blockers. 2,3-substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid-esters as potent mimics of dihydropyridines. Journal of Medicinal Chemistry, 33(9), pp.2629-2635.
CrossRef
- Mansouri, M., Movahedian, A., Rostami, M. and Fassihi, A., 2012. Synthesis and antioxidant evaluation of 4-(furan-2-yl)-6-methyl-2-thioxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate esters. Research in pharmaceutical sciences, 7(4), p.257.
- deVasconcelos, A., Oliveira, P.S., Ritter, M., Freitag, R.A., Romano, R.L., Quina, F.H., Pizzuti, L., Pereira, C.M., Stefanello, F.M. and Barschak, A.G., 2012. Antioxidant capacity and environmentally friendly synthesis of dihydropyrimidin‐(2H)‐ones promoted by naturally occurring organic acids. Journal of biochemical and molecular toxicology, 26(4), pp.155-161.
CrossRef
- Sharma, P., Rane, N. and Gurram, V.K., 2004. Synthesis and QSAR studies of pyrimido [4, 5-d] pyrimidine-2, 5-dione derivatives as potential antimicrobial agents. Bioorganic & medicinal chemistry letters, 14(16), pp.4185-4190.
CrossRef
- Trivedi, A.R., Bhuva, V.R., Dholariya, B.H., Dodiya, D.K., Kataria, V.B. and Shah, V.H., 2010. Novel dihydropyrimidines as a potential new class of antitubercular agents. Bioorganic & medicinal chemistry letters, 20(20), pp.6100-6102.
CrossRef
- Yadlapalli, R.K., Chourasia, O.P., Vemuri, K., Sritharan, M. and Perali, R.S., 2012. Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorganic & medicinal chemistry letters, 22(8), pp.2708-2711.
CrossRef
- Tale, R.H., Rodge, A.H., Hatnapure, G.D., Keche, A.P., Patil, K.M. and Pawar, R.P., 2012. The synthesis, anti-inflammatory and antimicrobial activity evaluation of novel thioanalogs of 3, 4-dihydrothiopyrimidin-2 (1 H)-one derivatives of N-aryl urea. Medicinal Chemistry Research, 21(12), pp.4252-4260.
CrossRef
- Adole, V.A., Pawar, T.B., Koli, P.B. and Jagdale, B.S., 2019. Exploration of catalytic performance of nano-La2O3 as an efficient catalyst for dihydropyrimidinone/thione synthesis and gas sensing. Journal of Nanostructure in Chemistry, 9(1), pp.61-76.
CrossRef
- Jubeen, F., Iqbal, S.Z., Shafiq, N., Khan, M., Parveen, S., Iqbal, M. and Nazir, A., 2018. Eco-friendly synthesis of pyrimidines and its derivatives: A review on broad spectrum bioactive moiety with huge therapeutic profile. Synthetic Communications, 48(6), pp.601-625.
CrossRef
- Adole, V.A., More, R.A., Jagdale, B.S., Pawar, T.B. and Chobe, S.S., 2020. Efficient synthesis, antibacterial, antifungal, antioxidant and cytotoxicity study of 2‐(2‐hydrazineyl) thiazole derivatives. ChemistrySelect, 5(9), pp.2778-2786.
CrossRef
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2020. Aqua‐mediated rapid and benign synthesis of 1,2,6,7‐tetrahydro‐8H‐indeno[5,4‐b] furan‐8‐one‐appended novel 2‐arylidene indanones of pharmacological interest at ambient temperature. Journal of the Chinese Chemical Society, 67(2), pp.306-315.
CrossRef
- Chobe, S.S., Adole, V.A., Deshmukh, K.P., Pawar, T.B. and Jagdale, B.S., 2014. Poly (ethylene glycol)(PEG-400): A green approach towards synthesis of novel pyrazolo [3, 4-d] pyrimidin-6-amines derivatives and their antimicrobial screening. Archives of Applied Science Research, 6(2), pp.61-66.
- Adole, V.A., Jagdale, B.S., Pawar, T.B. and Sagane, A.A., 2020. Ultrasound promoted stereoselective synthesis of 2,3-dihydrobenzofuran appended chalcones at ambient temperature. South African Journal of Chemistry, 73, pp.35-43.
CrossRef
- Adole, V.A., Waghchaure, R.H., Pathade, S.S., Patil, M.R., Pawar, T.B. and Jagdale, B.S., 2020. Solvent-free grindstone synthesis of four new (E)-7-(arylidene)-indanones and their structural, spectroscopic and quantum chemical study: a comprehensive theoretical and experimental exploration. Molecular Simulation, 46(14), pp.1045-1054.
CrossRef
- Adole, V.A., 2020. Synthetic approaches for the synthesis of dihydropyrimidinones/thiones (biginelli adducts): a concise review. World Journal of Pharmaceutical Research, 9(6), pp.1067-1091.
- Adole, V.A., Pawar, T.B. and Jagdale, B.S., 2021. DFT computational insights into structural, electronic and spectroscopic parameters of 2-(2-Hydrazineyl) thiazole derivatives: a concise theoretical and experimental approach. Journal of Sulfur Chemistry, 42(2), pp.131-148.
CrossRef
- Shinde, R.A., Adole, V.A., Jagdale, B.S., Pawar, T.B., Desale, B.S. and Shinde, R.S., 2020. Efficient Synthesis, Spectroscopic and Quantum Chemical Study of 2,3-Dihydrobenzofuran Labelled Two Novel Arylidene Indanones: A Comparative Theoretical Exploration. Material Science Research India, 17(2), pp.146-161.
CrossRef
- Zhao, S.Y., Chen, Z.Y., Wei, N., Liu, L. and Han, Z.B., 2019. Highly Efficient Cooperative Catalysis of Single-Site Lewis Acid and Brønsted Acid in a Metal–Organic Framework for the Biginelli Reaction. Inorganic chemistry, 58(12), pp.7657-7661.
CrossRef
- Patil, R.V., Chavan, J.U., Dalal, D.S., Shinde, V.S. and Beldar, A.G., 2019. Biginelli reaction: polymer supported catalytic approaches. ACS combinatorial science, 21(3), pp.105-148.
CrossRef
- Yao, N., Lu, M., Liu, X.B., Tan, J. and Hu, Y.L., 2018. Copper-doped mesoporous silica supported dual acidic ionic liquid as an efficient and cooperative reusability catalyst for Biginelli reaction. Journal of Molecular Liquids, 262, pp.328-335.
CrossRef
- Malik, A.A., Dangroo, N.A. and Ara, T., 2020. Microwave‐Assisted Tandem Kornblum Oxidation and Biginelli Reaction for the Synthesis of Dihydropyrimidones. ChemistrySelect, 5(42), pp.12965-12970.
CrossRef
- Gadkari, Y.U., Hatvate, N.T., Takale, B.S. and Telvekar, V.N., 2020. Concentrated solar radiation as a renewable heat source for a preparative-scale and solvent-free Biginelli reaction. New Journal of Chemistry, 44(20), pp.8167-8170.
CrossRef
- Narayanan, D.P., Sankaran, S. and Narayanan, B.N., 2019. Novel rice husk ash-reduced graphene oxide nanocomposite catalysts for solvent free Biginelli reaction with a statistical approach for the optimization of reaction parameters. Materials Chemistry and Physics, 222, pp.63-74.
CrossRef
- Wang, J. and Gu, H., 2015. Novel metal nanomaterials and their catalytic applications. Molecules, 20(9), pp.17070-17092.
CrossRef
- Daştan, A., Kulkarni, A. and Török, B., 2012. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green chemistry , 14(1) , pp.17-37.
CrossRef
- Kassaee, M.Z., Rostamizadeh, S., Shadjou, N., Motamedi, E. and Esmaeelzadeh, M., 2010. An efficient one‐pot solvent‐free synthesis of 2, 3‐dihydroquinazoline‐4 (1H)‐ones via Al/Al2O3 nanoparticles. Journal of Heterocyclic Chemistry, 47(6), pp.1421-1424.
CrossRef
- Safaei-Ghomi, J., Shahbazi-Alavi, H. and Heidari-Baghbahadorani, E., 2014. SnO nanoparticles as an efficient catalyst for the one-pot synthesis of chromeno [2, 3-b] pyridines and 2-amino-3, 5-dicyano-6-sulfanyl pyridines. RSC advances, 4(92), pp.50668-50677.
CrossRef
- Liu, X., Zhao, X., Zhu, J. and Xu, J., 2016. One‐pot synthesis of magnetic palladium–NiFe2O4–graphene oxide composite: an efficient and recyclable catalyst for Heck reaction. Applied Organometallic Chemistry, 30(5), pp.354-359.
CrossRef
- Xu, F., Lv, H.F., Wu, S.Y. and Ho-Pui, H.O., 2018. Light-activated gas sensing activity of ZnOnanotetrapods enhanced by plasmonic resonant energy from Au nanoparticles. Sensors and Actuators B: Chemical, 259, pp.709-716.
CrossRef
- Waghchaure, R.A., Koli, P.B., Adole, V.A., Jagdale, B.S.,Pawar T.B., 2020. Transition Metals Ni2+, Fe3+ Incorporated Modified ZnO Thick Film Sensors to Monitor the Environmental and Industrial Pollutant Gases.Oriental Journal of Chemistry,36(6), pp. 1049-1065
CrossRef
- Reen, G.K., Ahuja, M., Kumar, A., Patidar, R. and Sharma, P., 2017. ZnO Nanoparticle-catalyzed multicomponent reaction for the synthesis of 1, 4-diaryl dihydropyridines. Organic Preparations and Procedures International, 49(3), pp.273-286.
CrossRef
- Najar, A.H., Hossaini, Z., Abdolmohammadi, S. and Zareyee, D., 2020. ZnO-nanorods Promoted Synthesis of α-amino Nitrile Benzofuran Derivatives using One-pot Multicomponent Reaction of Isocyanides. Combinatorial chemistry & high throughput screening, 23(4), pp.345-355.
CrossRef
- Rostami‐Charati, F., Hossaini, Z., Zareyee, D., Afrashteh, S. and Hosseinzadeh, M., 2017. ZnO‐Nanorods as an Efficient Catalyst for the Synthesis of 1, 3‐Thiazolidine Derivatives by Aqueous Multicomponent Reactions of Isothiocyanates. Journal of Heterocyclic Chemistry, 54(3), pp.1937-1942.
CrossRef
- Rahman, Q.I., Ahmad, M., Misra, S.K. and Lohani, M., 2013. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Materials Letters, 91, pp.170-174.
CrossRef
- Kalpana, V.N., Kataru, B.A.S., Sravani, N., Vigneshwari, T., Panneerselvam, A. and Rajeswari, V.D., 2018. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano, 3, pp.48-55.
CrossRef
- Kadam, V.V., 2020. PEG-400 mediated highly efficient and benign one-pot synthesis of pyrimidone derivatives.World Journal of Pharmaceutical Research,9(5), pp.1062-1071
- Bukane, A.R. and Jagdale, B.S., 2021. DFT Exploration on Molecular Characteristics of 6-Methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Journal of Advanced Chemical Sciences, 7(1), pp.717-720.
CrossRef
- Singhal, S., Joseph, J.K., Jain, S.L. and Sain, B., 2010. Synthesis of 3, 4-dihydropyrimidinones in the presence of water under solvent free conditions using conventional heating, microwave irradiation/ultrasound. Green Chemistry Letters and Reviews, 3(1), pp.23-26.
CrossRef
- Nasr-Esfahani, M., Montazerozohori, M., Aghel-Mirrezaee, M. and Kashi, H., 2014. Efficient and green catalytic synthesis of dihydropyrimidinone (thione) derivatives using Cobalt nitrate in solvent-free conditions. Journal of the Chilean Chemical Society, 59(1), pp.2311-2314.
CrossRef
- Karade, H.N., Sathe, M. and Kaushik, M.P., 2007. Synthesis of 4-aryl substituted 3,4-dihydropyrimidinones using silica-chloride under solvent free conditions. Molecules, 12(7), pp.1341-1351.
CrossRef
- Sayyahi, S., Jahanbakhshi, S. and Dehghani, Z., 2013. A green and efficient method for the preparation of 3,4-dihydropyrimidin-2 (1H)-ones using quaternary ammonium-treated clay in water. Journal of Chemistry, 2013.
CrossRef
Views: 1,060
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal