J. Marshell
Department of Physics, Loyola College (Autonomous), Chennai - 600 034, India.
DOI : http://dx.doi.org/10.13005/msri/070128
Article Publishing History
Article Received on : 31 Dec 2009
Article Accepted on : 01 Feb 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Metal-free phthalocyanines(H2Pc) and its derivatives are an important organic electro and photo active material with remarkable chemical stability and flexibility offering considerable interest for many intensive applications. The complexity of the molecule makes the vibrational study of the Raman spectrum difficult. A vibrational assignment of the frequencies in the F.T Raman spectrum of H2Pc has been made in comparison with its metal derivatives, other related porphyrin, pyrrole, indole and ortho-subsituted benzene structures.
KEYWORDS:
H2Pc; Raman spectrum; Vibrational assignment
Copy the following to cite this article:
Marshell J. FT Raman Spectrum and Band Assignments for Metal–Free Phthalocyanine (H2Pc). Mat.Sci.Res.India;7(1)
|
Introduction
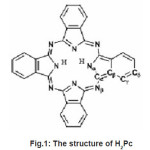
Phthalocyanines are porphyrin derivatives that are characterized by a high degree of symmetry, planarity and electron delocalization. Phthalocyanines, has received considerable attention as charge generating materials in electrophotographic receptors, in solar cells, as organic semiconductors, in optical-data storage and in non-linear optics. Phthalocyanines are a class of materials which is most extensively investigated because of their great structural flexibility, and can host ~70 different elements in the central phthalocyanine cavity and also a large range of peripheral substituents of phthalocyanines is known to improve the poor solubility of the unsubstituted phthalocyanine. They consist of isoindole rings at the four corners linked together by four nitrogen atoms. The middle is a 16 membered ring called the macrocycle in which the two imino-hydrogen atoms are replaceable by metal. The space within the four central nitrogen atoms when occupied by hydrogen atoms become metal- free phthalocyanine or H2Pc and when by metallic ions having coordination number greater than four to become metal substituted phthalocyanine or MPc. The metal atom is held by the two isoindole N atoms of H2Pc by primary valencies and is coordinated by the other two N atoms of the macrocycle to form four chelate rings adding greater stability to the structure than H2Pc. Metal-free phthalocyanine (H2Pc) is known to exist in polymorphic forms of the types; face centred monoclinic β-H2Pc, the tetragonal α-H2Pc, and the hexagonal x-H2Pc and τ-H2Pc which are intermediate between α and β H2Pc, all differing substantially in electronic conductivity and photoactivities. The structure of H2Pc is presented in Fig.1. The polymorphs have different stack arrangement and different stacking overlap of adjacent molecules. The electronic properties are related to the overlap of electron wavfunctions and the stacking habit of molecules. The α-H2Pc has a large intermolecular P wavefunction overlap resulting in a large carrier mobility. The intermolecular interaction and the stacking habit of molecules are considered to affect low wave-number Raman spectra in α-H2Pc which exhibits two peaks at 90cm-1and 132 cm-1 while other polymeric forms of H2Pc have only one band at 132cm-1 that have not been identified with a characteristic vibrational mode.1
Figure 1: The structure of H2Pc
Experimental
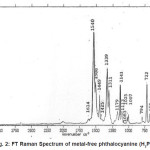
H2Pc crystals were prepared by vacuum sublimation under a pressure of about 10-5 torr and at a temperature of about 460°c.The crystals are formed as whiskers having dimensions of 15-20×0.3×0.13 mm.The FT Raman spectrum of H2Pc was recorded on Bruker IFS 66v spectrophotometer using a FRA106 Raman module and a 200mW YAG laser is presented in Fig. 2.
Figure 2: FT Raman Spectrum of metal-free phthalocyanine (H2Pc)
Result and Discussions
The molecular symmetry of H2Pc is D2h while its metal derivatives have D4h symmetry. The reduced symmetry of H2Pc is concluded on the basis of Eu mode of vibration which is infrared active for the D4h symmetry cases.The Eu mode splits into two other infrared active modes B2u and B3u without too much change in the peak position when the molecule assumes D2h symmetry . The rule of mutual exclusion applies and only the gerade modes appear in the Raman spectrum.
The vibrations of phthalocyanines are regarded as complex but can be divided into two main types; higher frequency vibrations mainly due to the motion of the isoindole rings modified by the macrocycle and the other low frequency vibrations of the metal-nitrogen bonds of MPc’s. It has been observed that absorption bands change with the coordination of the metal ion with the four nitrogen atoms of the macrocyclic ring leading to a classification of vibrations as metal dependent and metal independent. The probable assignments of Raman frequencies for the H2Pc is given in Table 1.
Table 1: Raman band assignments for H2Pc
The characteristic frequencies for the inner macrocycle are observed at 132 cm-1, 231 cm-1, 681 cm-1, 722 cm-1 and 794 cm-1 in the Raman spectrum. The 681cm-1 corresponds to a motion in which the co-ordinated nitrogen atoms breadthe symmetrically in a typical macrobreathing vibration while all the other vibrations correspond to a macrocycle ring deformation. The vibrations of isoindole group have been assigned in comparison with the vibrations of pyrrole and indole groups.2-4
The isoindole and pyrrole stretches have been assigned to 1449cm-1,1425cm-1 and 1540 cm-1,1508cm-1, 1339cm-1, 1141cm-1, 1113cm-1 respectively . The motions of the isoindole rings have a greater effect on the macrocycle. The CNC group stretch at 1540cm-1 is the most intense bands arising from Cα-Nα and Cα-Nβ stretches that cause a significant alterations of the macrocycle. This Raman band is highly sensitive to the metal ion and the cavity size in MPc’s. In ZnPc the metal ion is greater than the cavity size (3.96A°) and the CNC stretch occur at 1505 cm-1 while the cavity size for NiPc is 3.66A° giving the corresponding peak postion at 1545cm-1 and being the highest in all phthalocyanines. There are a number of in-plane skeletal pyrrole ring vibrations in the porphyrin system which are analogous to the phthalocyanine isoindole ring vibrations. The frequencies at 567cm-1, 480cm-1, and 189cm-1 have been assigned to the isoindole ring deformations. The 480cm-1 frequency is a complex vibration involving the isoindole rings.5-10
Boucher and Gatz11 have reported that the N-H deformations of photoporphyrins give rise to sharp absorptions bands at 1110cm-1 and 740cm-1 which have been observed in the Raman spectrum of H2Pc at 1007cm-1 and 722cm-1 respectively.The significant comparative shift in the frequencies is attributed to the effect of C-H bending and macrocycle deformation vibrations of H2Pc. A very broad band of low intensity has been observed at 3420 cm-1 and has been assigned to the weakly bonded O-H stretching of crystalline water which usually give weak Raman bands.
The stretching and deformation vibrations of the C-C and C-H bonds of the external rings are metal independent and they have been assigned in comparison wth ortho- substituted benzene 12,13. The 3058cm-1 band is assigned to all C-H stretchings while the band at 1614 cm-1 is assigned to C-C ring stretches. The C-H in-plane bendings occur at frequencies 1179cm-1,1141cm-1and 1083cm-1 while the C-H out-of-plane bendings occur at 1025cm-1 and 1007cm-1. The vibrations at 567cm–1 and 542cm-1 have been assigned to C-C ring in-plane deformations.
In crystalline napthalene which belongs to the same space group, lattice vibrations were observed below 100cm-1 and so the vibration at 132cm-1 is a macrocyle ring deformation mode. Further the Raman spectra shows no combination bands in the region 1800-3000 cm-1 whereas all metal Pc’s exhibit overtone and combination bands in this region.
Conclusion
The Fourier Tranform Raman spectrum of H2Pc has been investigated in comparison with other metal phthalocyanines, porphyrin, pyrrole, indole and benzene derivatives for the observed frequencies in the spectrum and a complete assignment of Raman bands is proposed for H2Pc.
References
- Leznoff C.C, Lever A.B.P, Phthalocyanines-Properties and Applications, vol.1, VCH New York, (1989): vol.3 (1993).
- Suiwaiyan A. and Zwarich R., Spectrochim Acta, 42A: 1017 (1986).
CrossRef
- Majaoube M., Vergoten G., J. Raman Spectrosc., 28(8): 431 (1992).
CrossRef
- Takeuchi H. and Harada I., Spectrochim Acta, 42A: 1069 (1986).
CrossRef
- Nakamoto K., Infrared and Raman Spectra of Inorganic and Coordination compounds (PartB), P-41,Wiley-VCH New York, (2009).
- Shruvell H.F and Pinzuiti L., Can. J. Chem., 44: 125 (1966).
CrossRef
- Aroca R., Dilella D.P and R.O Loutfy, J. Phys.Chem.(solids), 43(8): 707 (1982).
- Jennings C.A, Aroca R., Kovacs G.J, Hsaio C., J.Raman spectrosc., 27: 867 (1996).
CrossRef
- Tackley D.R, Dent G. and Smith W.E,Phys.Chem.Chem. Phys., 2: 3949 (2000).
CrossRef
- Dent G. and Farell F., Spectrochim Acta, 53A: 21 (1997).
- Boucher L.J and Katz J.J, J.Am.Chem. Soc., 89: 1340 (1967).
CrossRef
- Green J.H.S, Kynaston W. and Paisley H.M, J. Chem. Soc., 473 (1963).
CrossRef
- Scherer J.R and Evans J.C, Spectrochim Acta, 19: 1739 (1963).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal