S. Panda1, G. C. Mohanty2, R.N. Samal3 and G. S. Roy4
1Department of Physics, S.B. Woman's College, Cuttack - 753 001, India.
2Department of Physics, G.M. (Auto) College, Sambalpur - 768 004, India.
3Department of Physics, Ravenshaw University, Cuttack - 753 003, India.
4Plot No. 6, Budheswari Colony, Bhuneswar - 751 006, India.
Corresponding Author E-mail: sarojinipanda21@gmail.com
DOI : http://dx.doi.org/10.13005/msri/070214
Article Publishing History
Article Received on : 18 Oct 2010
Article Accepted on : 14 Nov 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
Reduced viscosity (ηsp/C) and inherent viscosity ln (ηrel/C) of PVA (Mw = 1,25,000) has been calculated by measuring the flow time of polymer solution in solvents like distilled water and 4M Urea at six different tempratures 25° C, 30° C, 35° C, 40° C, 45° C, and 50° C. From exptrapolation of curve (/C) versus C and (ln /C) versus C, thermoviscosity parameters like Huggins’ Constrant (KHl), Kraemer's constant (KHll) and viscosity concentration co-efficient (a2) have been estimated. In aqueous solution (PVA in distilled water), Huggins' relation does not hold good. So a2 = .201[h]2.28 is used; but in aqueous Urea (PVA in 4M Urea), Huggins' relation holds good. Also η =KMα and value of a more for 4M Urea i.e aqueous Urea is better solvent for PVA than distilled water.
KEYWORDS:
Polyvinyl alcohol; Distilled water; Urea
Copy the following to cite this article:
Panda S, Mohanty G. C, Samal R. N, Roy G. S. Viscosity Studies of Polyvinyl Alcohol (PVA, Mw = 1,25,000) in Solvent Distilled Water and Aqueous Solution of Urea. Mat.Sci.Res.India;7(2)
|
Copy the following to cite this URL:
Panda S, Mohanty G. C, Samal R. N, Roy G. S. Viscosity Studies of Polyvinyl Alcohol (PVA, Mw = 1,25,000) in Solvent Distilled Water and Aqueous Solution of Urea. Mat.Sci.Res.India;7(2). Available from: http://www.materialsciencejournal.org/?p=2400
|
Introduction
Viscosity measurements of polymer solution shows the existence of molecular interaction between the polymer and the solvents. From these measurements, the extent of interaction can also be predicted. Solvation dynamics1,2 and its experimental and theoritical studies have given rise to renewed interest in the field of research activities.3-6
Several studies of the viscosity concentration relation of PVA in dilute aqueous solutions have been reported7 earlier, but their results do not agree and the causes of this discrepancy are unknown.
In the present study, the viscosity-concentration relation for PVA at six direfferent tempratures are calculated. Generally, distilled water is used as solvent for PVA (Mw = 1,25,000) but since it is a rather poor solvent, measurements are also made with solvent 4M Urea, which is expected to be a good solvent.6
Experimental
Requisites
Polymer
The polymer PVA of molecular weight (Mw = 1,25,000) is used as such without further purification.
Solvents
The solvents are distilled water and aqueous solution of (Urea BDH (AR) grade); used as such through out the expirement. A freshly prepared solution (1wt %) of the sample is prepared in solvent 4M Urea and Distilled water.
Viscometer
The viscocities of polymer solution as well as solvent were determined by a viscometer. We used Ubbelohde Suspended level viscometer (USLV) for our study. It is a simple glass capillary device. It is designed in such a manner that the measurement is unaffected by the volume of the solution taken.
The conditions for preparing aqueous solution of PVA are important, because they may exert a remarkable influence on the solution viscosity. When the dissolving time is less than 1 (one) hour at 100°C, the viscosity is highly time-dependent. When the time is more than 2 hours, the viscosity shows little change, but decreses gradually with time. This decrese can be ignored for time less than 5 hours. In our experiment, the dissolving time at 60°C is 8-10 hours and was standardised at about 1-2 hours at 100°C.
Results and Discussion
In aqueous solution of PVA (Mw =1,25,000), plots of hsp/C ~ C and (ln hrel/C ~C are straight lines (Fig. 1). In aqueous Urea both /C and (ln )/C versus C also show the ordinary rectilinear relations (fig.2). But the slope of (ln )/C versus C plot is negative in case of a aqueous Urea, where as it is positive in case of solvent distilled water.
Figure 1: hsp/c (a,b,c,d,e,f) and hrel/C (a’,b’,c’,d’,e’,f’) plotted against concentration (c) for PVA (MW=1,25,000) in distilled water at different temperature
Figure 2: hsp/c (a,b,c,d,e,f) and hrel/C (a’,b’,c’,d’,e’,f’) plotted against concentration (c) for PVA (MW=1,25,000) 4M Urea at different temperature
The specific viscosity of the polymer solutions10,11 may be generally expressed as:
ηsp= a1c + a2c2 + a3c3 …(1)
Considering the dilute solutions, terms higher than the second order are neglected, giving
ηsp/C= a1 + a2C …(2)
According to Hunggins,11
a2 = KHl[η]2 …(3)
So that ηsp/C =[η] + KH‘[η]²C …(4)
Values of a2 and KHl estiamted from the viscosity measurements of PVA (Mw = 1,25,000) in aqueous solution and in 4M Urea are shown in Table 1 and 2.
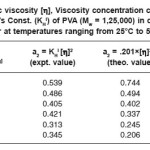
Table 1: Intrinsic viscosity [h], Viscosity concentration co-efficient (a2), Huggins’s Const. (KHl) of PVA (Mw = 1,25,000) in distilled water at temperatures ranging from 25°C to 50°C
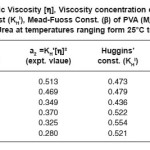
Table 2: Intrinsic Viscosity [h], Viscosity concentration co-efficient (a2), Huggins’ const (KHl), Mead-Fuoss Const. (b) of PVA (Mw = 1, 25, 000) in 4M Urea at temperatures ranging form 25°C to 50°C.
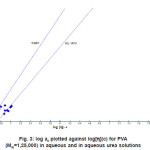
The results appear in Fig. 3, where log a2 is plotted against log [h] for both aqueous and aqueous Urea solutions. In each case, a rectilinear relation is well realised, but the slope differs in each system. In aqueous Urea, the slope is a2 =KH‘[h]², according to Huggins’ formula; but in aqueous solution the slope is higher, corresponding to
a2 = 0.201 × [η]2.28 …(5)
The evaluated values of a2 from eq. (5) are shown in Table 1 and are in good agreemnet with experimental values.
Figure 3: log a2 plotted against log[h](c) for PVA (MW=1,25,000) in aqueous and in aqueous urea solutions
In the aqueous urea, the Mead Fuoss euation12
ln ηrel/C = [η]-β[η]²C is in good agreement with experiment. Some examples of b are illustrated with KHl in Table 2. The sum of KHl and b (Mead-Fuoss const.) agree well with the theoritical value 0.5.
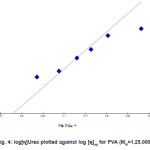
The relation between the intrinsic viscosity in the aqueous Urea and in aqueous solution is illusatrated in a log–log plot given in fig. 4.
Figure 4: log[h]Urea plotted against log [h]aq for PVA (MW=1,25,000)
From fig. 4, the following emperical formula is obtained
[h]
Urea=1.7[h]aq
1.164
In which [h]
Urea and [h]
aq are the intrinsic viscosities of PVA (M
w=1,25,000) in the aqueous Urea and in the aqueous solution, respectively. This means that, the exponent a of the Sakurada-Houwink equation, [h]= KM
a, is higher in the aqueous Urea than in the aqueous solutions i.e. the aqueous Urea is a better solvent than water.
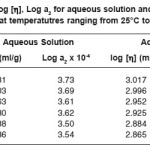
Table 3: Log [η], Log a2 for aqueous solution and aqueous Urea at temperatutres ranging from 25°C to 50°C
Acknowledgments
One of the Authors, S. Panda, has expressed her whole hearted thanks to Dr. (Mrs.) Rita Paikray, HOD,Dept of Physics, Ravenshaw University, Cuttack, Orissa for providing Laboratory facilities during this investigation.
References
- Roy S, Momath S and Bagchi B, Proc Indian Acad Sci Chem Sci, 79: 105(1993).
- Bagchi B, J chem Phys, 95: 93, 467 (1991).
- Chandra A and Bagchi B, J Phys Chem, 93: 6996 (1989).
- Huggins M L, J Am Chem Soc, 64: 2716 (1994).
- Dash U.N., Roy G.S., Talukdar M, Moharatha D, Ind. Jour., of Pure and Applied Physics . 48: 651 (2010).
- Dash U.N., Roy G.S., Moharatha D, Talukdar M., Physics and Chemistry of liquids(In press)
- A.Nakajima and K.Furudachi. Chem.High Polymer(Japan), 6: 460 (1949)
- Masakazu Matsumoto and Kiyokazu Imai, J.Polymer Sci. 24: 125-131 (1957).
- Mahapatra A.P., Samal R.K., Samal, R.N. and Roy G.S., Ultra Scientist of Phys. Sci,11(1): (1999).
- W.R. Krighaum and F.T. Wall. J. Polymer Sci. 5: 505 (1950).
- M.L. Huggins, J.Am. Chem, Soc 64: 2716 (1972).
- D.F. Mead, and R.M. Fuoss, J. Am. Chem.Soc, 64: 277 (1912).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal