Ratna Kartikasari1, Soekrisno2, M. Noer Ilman2 and Suyitno2
1Department of Mechanical Engineering, Technical Faculty, Gadjah Mada University Jl. Grafika No.2 Kampus UGM, Depok, Sleman, Yogyakarta - 55281, Indonesia.
DOI : http://dx.doi.org/10.13005/msri/070112
Article Publishing History
Article Received on : 20 Apr 2010
Article Accepted on : 24 May 2010
Article Published :
Plagiarism Check: No
Article Metrics
ABSTRACT:
On the basis of economic considerations, Fe-Al-C alloy could be a good candidate for replacing some of the conventional stainless steel. Wherein, Al is used to replace the expensive alloy element in conventional Fe-Cr-C system. The aim of the research is to investigate harden ability and corrosion resistance of Fe-9Al-0,6C in the NaCl and H2SO4 solution. Thirty five kilograms of Fe-Al-C were prepared from mild steel scrap, high purity aluminium, and Fe-C. The alloy was prepared in an induction furnace under an argon atmosphere. Jominy test and microstructure were examined. And, the corrosion rate, were carried out with three-electrode polarization in 0.5% NaCl and 0.05 M H2SO4. The corrosion type and the morphology of the oxide scale were examined by optical microscopy and scanning electron microscope (SEM). Corrosions products were examined with EDS/EDAX. The optical micrograph shows that as cast Fe-9Al-0,6C alloy has ferrite and pearlite microstructure. The result of Jominy test shows that these alloy non-harden able. The result of corrosion testing showed that the alloy more resistance in NaCl than in H2SO4 solution. Optical micrograph and SEM analysis indicated that corrosion form is general corrosion and there is no trend toward intergranular attack.
KEYWORDS:
Fe-Al-C alloy; Conventional stainless steel; Harden ability; Corrosion resistance
Copy the following to cite this article:
Kartikasari R, Soekrisno, Ilman M. N, Suyitno. Hardenability and Corroson Resistance of as Cast Fe-9Al-0,6C Alloy. Mat.Sci.Res.India;7(1)
|
Copy the following to cite this URL:
Kartikasari R, Soekrisno, Ilman M. N, Suyitno. Hardenability and Corroson Resistance of as Cast Fe-9Al-0,6C Alloy. Mat.Sci.Res.India;7(1). Available from: http://www.materialsciencejournal.org/?p=2238
|
Introduction
Fe-Al-C alloy is being developed for elevated temperature structural application up to 873K.2-4 In addition, the plain iron-aluminum lightweight steel containing up to 9 wt-% Al shows a reduction in density of 10% and more¹. On the basis of economic and lower density considerations, this material could be a good candidate for replacing some of the conventional stainless steel.5-6 Wherein, Al is used to replace the expensive alloy element (Cr) in conventional Fe-Cr-C system.
Ferritic iron aluminum is show promising physical, mechanical and technological properties, corrosion and oxidation resistance, and low raw material cost2 which is suitable for development and design of new type of high strength lightweight steel.3 In addition, the plain iron-aluminum lightweight steel containing up to 9 wt-% Al shows a reduction in density of 10% and more.
In contrast, Fe-Al alloys exhibit poor toughness and brittle behavior at room temperature. Recent studies indicate that reduction in Al content to 8.5 wt-% resulted in enhanced ductility. In previous work, it has been shown that low carbon content (0.05 and 0.1 wt-%) in Fe-9 wt-% Al leads low tensile ductility.7 Addition of carbon to Fe-Al containing 8.5 to 16 wt-%Al leds to higher strength,5 and better machinability.6 It has been shown that low carbon content (0.05 and 0.1 wt-%) in Fe-9 wt-% Al leads low tensile ductility.7 Whereas, the ESR ingots of Fe-10.5Al and Fe-13Al alloys containing high (0.5 and 1.0 wt-%) carbon exhibit excellent hot workability.8 The Fe-10.5 wt-%Al and Fe-13 wt-%Al alloys with low carbon content (0.05 and 0.1 wt-%) exhibit the presence of fine needle shaped Fe3AlC0.5 precipitates in the matrix and along the grain boundaries.8
Fe-Al-C alloy is being developed for elevated temperature structural application up to 873K.9,10
Aluminum plays a major role in the oxidation and corrosion resistance which is characteristic of the binary Fe-Al alloy.11 The Fe-Al-C alloys have good corrosion resistance in a neutral environments and were corrode at rates comparable to that of white cast-iron in acid environments.12 However, few data are available on the corrosion phenomena of Fe-Al alloy in acid media like H2SO4.
In this study, harden ability and corrosion behavior of Fe-9Al-0.6C alloy have been reported.
Material and Methods
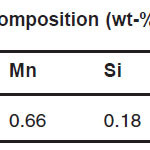
Thirty five kilograms of Fe-Al-C were prepared from mild steel scrap, high purity aluminum, and Fe-C. The alloy was prepared in a high frequency induction furnace under a controlled protective argon atmosphere. The chemical compositions are listed in Table 1. The ingot was cut using bimetallic band saw blade to make the Jominy specimen (EN ISO 642 standard) and the corrosion specimen (14 mm in gauge diameter and 3 mm in gauge length). Microstructures of as cast alloys were examined by using an optical and electron microscope. The possible phases present in the diffraction specimens were identified using X-ray diffraction with a copper target, nickel filter and a graphite single crystal monochromater.
Table 1: Chemical composition (wt-%) of the alloy tested
The surface of the corrosion specimens were mechanically polished with abrasive paper up to 1200 grit, after surface finishing. The last mechanical polishing was done with 0.5 5µm alumina paste. The corrosion rate were carried out with three-electrode polarization in 0.5% NaCl and 0.05M H2SO4. The corrosion type and the morphology of the oxide scale were examined by optical microscopy and scanning electron microscope (SEM). Corrosions product were examined with EDS/EDAX.
The polished section were subsequently etched with 3.3% HNO3-3.3% CH3COOH-0.1% Hf-93.3% H2O by volume for micro structural examination by optical microscope.
Results and Discussion
Harden Ability Analysis
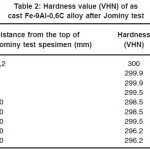
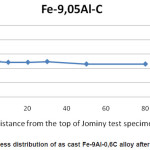
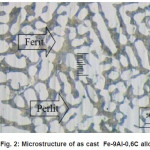
Table 2 shows the hardness value after Jominy test. Figure 1 shows hardness distribution of Fe-9Al-0.6C alloys curve after Jominy test. There is no significantly increasing hardness. Figure 2 shows the microstructure of as cast Fe-9Al-0.6C alloy. The as cast Fe-9Al-0.6C alloy is composed of α-Fe and pearlites. It has been known that Al was stabilized ferrite phase [10] but appearance of pearlites structure because of the content of C.
Table 2: Hardness value (VHN) of as cast Fe-9Al-0,6C alloy after Jominy test
Figure 1: Hardness distribution of as cast Fe-9Al-0,6C alloy after Jominy test
Figure 2: Microstructure of as cast Fe-9Al-0,6C alloy
Figure 3 shows the microstructure of as cast Fe-9Al-0.6C alloys after Jominy test. The as cast Fe-9Al-0.6C alloy microstructures are full ferritic. Pearlite structure is decomposed to ferrite structure after heating and then rapid cooling on the Jominy test. Because of cooling rate differentiation, microstructure of as cast Fe-9Al-0.6C alloy differents from the top toward to the end of Jominy test specimen only on the magnitude of grain. High Al content of as cast Fe-9Al-0.6C alloy caused ferrite structure stabilized. Heating of specimen was continued with quenching no change ferrite structure to martensite because of high Al content. Al was known as ferrite stabilizer, although this alloy has high C (0.6 wt%) that is not influence ferrite stabilizer. So, that is conformity between hardness distribution and microstructure after Jominy test. It can concluded that as cast Fe-9Al-0.6C alloy is non harden able with heat treatment.
Figure 3: Microstructure of the as cast Fe-9Al-0,6C alloy after Jominy test
Corrosion Resistance Analisys
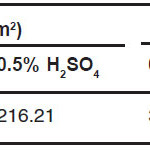
The three-electrode polarization was used to examine corrosion rate of the alloy. The result of the examination was showed in table 2. Table 2 indicates that in the 0.5% NaCl solution the corrosion rate of the as cast Fe-9Al-0.6C alloy is lower than in the 0.05 M H2SO4 solution. The differentiation of the corrosion rate is caused solution reactivity. This corrosion rate is fair category in the 0.5% NaCl solution and poor category in the 0.05 M H2SO4 solution.14
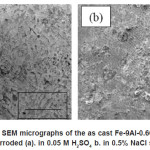
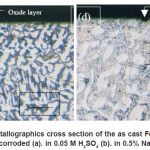
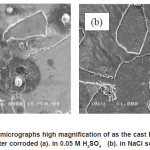
Figur 4. shows surface corroded for the alloys. Light and dark pattern microstructures seem at this picture. Whereas, in the higher magnification (Figur 6) oxide pattern was very clear. SEM-EDS analysis (Figur 7) shows present some oxides like Al2O3 and FeO that are come from composition of these alloy, while Na2O, SO3 and Cl are from corrosion medium. The micrograph cross section of as cast Fe-9Al-0.6C alloy after corroded show that the oxide layers formed on the surface of the alloy after corrosion testing (Fig 5). It was observed that thin oxide layer formed on the surface but internal oxidation product could not be observed. The thickness of oxide layer of as cast Fe-9Al-0.6C alloy could be observed in both of corrosion medium. These phenomena connected with corrosion rate. General corrosion could be observed on the surface of corrosion specimen but no intergranular attack could be observed in these conditions.
Figure 4: SEM micrographs of the as cast Fe-9Al-0.6C alloy after corroded (a). in 0.05 M H2SO4 b. in 0.5% NaCl solution
Figure 5: Metallographics cross section of the as cast Fe-9Al-0.6C alloy after corroded (a). in 0.05 M H2SO4 (b). in 0.5% NaCl solution
Figure 6: SEM micrographs high magnification of as the cast Fe-9Al-0.6C alloy after corroded (a). in 0.05 M H2SO4 (b). in NaCl solution
Figure 7: SEM micrographs of the as cast Fe-9Al-0.6C alloy after corroded (a). SE image in 0.05M H2SO4 0.05M (b). SE image in 0.5% NaCl c. EDS result in 0.05M H2SO4 0.05M d. EDS result in 0.5% NaCl solution
Table 3: Corrosion rate of the as cast Fe-9Al-0.6C alloy
Conclusions
The as cast Fe-9Al-0.6C alloy has ferrites and pearlites microstructure. After Jominy test, microstructure this alloy change into full ferritic. The as cast Fe-9Al-0.6C alloy is non harden able with heat treatment. General corrosion could be observed after three-electrode polarization but there is no trend toward to intergranular corrosion attack.
References
- Huang, Y.D., and Froyen, L., Recovery, Recrystalization, and Grain Growth in Fe3Al Based alloys, Intermetallics. 10: 443 (2006).
- Kobayashi, S., Zaefferer, S., Schneider, A., Raabe, D., and Frommeyer, G., Optimisation of Precipitation for Controlling Recrystallization of Wrought Fe3Al Based Alloys, Intermetallics, 13: 1296-1303 (2005).
CrossRef
- Frommeyer, Physical and Mechanical Properties of Iron-Aluminium-(Mn-Si) Lightweight Steels, The 1999 ATS International Steelmaking Conference, Paris. Sec.4, (2000).
- Jablonska, M., Rodak, K., and Niewielski, G., Characterization of The Microstructure of FeAl alloy after Hot Deformation, Journal of Achievements in Materials and Manufacturing Engineering. 18(1): 107-110 (2006).
- Baligidad, R.G., Prakash, U., Ramakrishna Rao, V., Rao, P.K., and Ballal N.B., Effect of Carbon Content on Mechanical Properties of Electroslag Remelted Fe3Al Based Intermetallic alloys, ISIJ, 36(12): 1453-1458 (1996).
CrossRef
- Baligidad, R.G., Prakash, U., and Radha Krishna, Effect of Carbon Addition on Structure and Mechanical Properties of Electroslag Remelted Fe-20wt.%Al alloy, 249(1-2): 97-102 (1998).
- Stollof, N.S. and Liu, C.T., Review: Environmental embrittlement of Iron Aluminides, Intermetallics, 2, pp. 75-87, (1994).
CrossRef
- Baligidad, R.G. and Satya Prasad, K., Effect of Al and C on Structure and Mechanical Properties of Fe-Al-C alloys, Mater. Sci. Technol., 23(1): 38-44 (2007).
CrossRef
- Sikka, V.K., Viswanathan, S., and McKamey, C.G., in ‘Structural Intermetallic’, (ed. R. Darolia et al.), Warrendale, PA, TMS. pp. 483-491 (1993).
- Prakash, U. Buckley, R.A., Jones, H. and Sellars, C.M., Structure and Properties of Ordered Intermetallics Based on the Fe-Al System, ISIJ Int., 31(10): 1113-1126 (1991).
CrossRef
- Kao, C.H., and Wan, C.M., Effect of Temperature on the Oxidation of Fe-7.5Al-0.65C alloy, J. Mater. Sci., 23, 1943-1947, (1988).
CrossRef
- Giza, K., Bala, H., Wysocki, J.J., and Szymura, S., Corrosion Resistance of the Fe-Al-C Permanent Magnet Alloy, Intermetallics, 6(5): 357-362 (1998).
CrossRef
- Bastin, G.F., Van Loo, F.J.J., Vrolijk, J.W.G.A., and Wolf, L.R., Crystallography of Aligned Fe-Al Eutectoid, Journal of Crystal Growth, 43: 745-751 (1978).
CrossRef
- Fontana, G.M., Corrosion Engineering, 3th ed., McGraw Hill Inc. Singapore, 153-18,1(1987).

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal